Multi-Modality Imaging
Dual-Modality Imaging with SPECT/CT
Written in 2004
- Bruce Hasegawa, PhD
- Randall A. Hawkins, MD, PhD
Medical diagnoses commonly rely on assessment of both the functional status and the anatomical condition of the patient. In a clinical setting, in vivo measurement of organ physiology, tissue metabolism, tissue perfusion, and other biological functions can be performed with radionuclide-tracer techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). However, radionuclide imaging has relatively poor spatial resolution, often is photon starved, and can lack anatomical cues that are needed to localize or stage the disease. In comparison, imaging methods such as computed tomography (CT) or magnetic resonance imaging (MRI) have excellent spatial resolution, are rich in anatomical detail, but have limited functional content.
To facilitate the process of correlating structural and functional information, investigators at UCSF and elsewhere have developed a new class of diagnostic instrumentation that combines X-ray CT and radionuclide imaging with SPECT or PET. These "dual-modality" systems use separate detectors for X-ray and radionuclide imaging, with the detectors integrated on a common gantry to simplify patient handling, data acquisition, and co-registration of the CT and radionuclide image data. The CT and radionuclide data are acquired with the patient in the same position to facilitate image correlation between the CT and SPECT or PET data. This makes it possible to produce a "fused image" in which the radionuclide distribution can be displayed in color on a gray- scale CT image to co-register the anatomical and physiological features and thereby improve evaluation of disease, in comparison to SPECT or PET alone.
In addition, dual-modality imaging provides another benefit: that of improving quantitative functional assessments obtained with SPECT or PET. Specifically, conventional radionuclide images can be compromised by soft-tissue attenuation and by other physical effects such as scattered radiation. In dual-modality imaging systems, the CT data can be used to generate a patient-specific map of attenuation coefficients to correct the radionuclide image for photon attenuation. In this way, dual-modality imaging can improve both anatomical localization and quantitative accuracy of radionuclide imaging, and therefore it is gaining acceptance and is being adopted worldwide for clinical use.
Clinical Implementation
|
The conceptual design of dual-modality imaging systems is quite simple. A typical dual-modality imaging has a CT scanner (X-ray tube and detector) and a radionuclide detector (PET or SPECT) mounted on a single gantry with a common patient table for X-ray and radionuclide imaging. CT and radionuclide scans are acquired by translating the patient from one detector to the other while the patient remains on the patient table. This allows the CT and radionuclide images to be acquired with a consistent scanner geometry and body habitus, and with minimal delay between the two acquisitions. After both sets of images are acquired and reconstructed, image registration software then is used to fuse the X-ray and radionuclide images in a way that accounts for differences in scanner geometry and image format (e.g., 128_128 vs. 512_512) between the two data sets. At UCSF, researchers in the Department of Radiology, and specifically at its Physics Research Laboratory, pioneered the development of dual-modality imaging systems that combine X-ray CT and SPECT. The concept of dual-modality imaging at UCSF was conceived by Christopher Cann, PhD, Robert Gould, ScD, and Bruce Hasegawa, PhD, faculty members in the Physics Section of the Radiology Department at UCSF, and by Eric Gingold, PhD, a graduate student in Bioengineering at UCSF. Over the past decade, research with dual-modality imaging at UCSF involved theoretical modeling (Soo Chin Liew, PhD), computer simulations (Susan M. Reilly, MS), and development of the first prototype scanner (Thomas Lang, PhD). Implementation of a second prototype scanner included development of image reconstruction techniques (J. Keenan Brown, PhD), and of new application-specific integrated circuits and detector characterization experiments (Joseph Heanue, PhD). These developments led to quantitative measurements of radionuclide uptake in a porcine model of myocardial perfusion (Kathrin Kalki, PhD) with the prototype scanner. The development of these prototype systems led to the implementation of the first human-scale dual-modality system for clinical research (Figure 1) at UCSF (Stephen Blankespoor, MS) in collaboration with GE Medical Systems. The system was configured by siting a scintillation camera (GE 600 XR/T) adjacent to a commercial CT scanner (GE 9800 Quick), allowing the patient to be scanned first in the CT scanner, then moved into the SPECT system by simple translation of a common patient table. The first studies with this newer system included quantitative radionuclide assessments of myocardial perfusion (Angela J. Da Silva, PhD), again in a porcine model, and followed by the development of techniques for assessing cancer (H. Roger Tang, PhD), and for diagnosis of prostate cancer patients with CT/SPECT (Kenneth H. Wong, PhD). Additional experimental studies have included development of new detectors and detector electronics (Koji Iwata, PhD, William Barber, PhD).
|
Dual-Modality Imaging at UCSF
|
GE Medical Systems (Milwaukee, WI) and Elgems Ltd (Haifa, Israel) have developed a dual-modality imaging system ("Millenium VH" or "Hawkeye") that combines SPECT and CT, similar to the approach developed at UCSF. The system performs planar scintigraphy or SPECT, and also performs coincidence detection of annihilation photons for imaging 18F-fluorodeoxyglucose (FDG) and other positron- emitting radionuclides, and incorporates a low-resolution CT scanner for anatomical localization and attenuation correction of the radionuclide data. The Hawkeye system now is installed in the Nuclear Medicine Clinic at Moffitt Long Hospital (Figure 2). It is interfaced both into the Radiology PACS system and into the Nuclear Medicine local area network. This makes it possible to view images from the Hawkeye directly on the GE Integra workstation next to the camera, or on an additional Integra workstation in the Nuclear Medicine view room, and also on PACS terminals and on the Nuclear Medicine Pegasys (Philips ADAC) workstations. The system is used for both clinical and research studies. It has been used for routine clinical procedures (Figure 3) such as bone, renal and cardiac studies as well as for selected FDG coincidence imaging procedures. Single slice CT images having a slice width of 10 mm can also be acquired with the unit, and it is expected that in the future most nuclear medicine clinical studies will benefit from this level of co-registered CT acquired with nuclear medicine image sets. Areas of particular relevance of CT to clinical interpretation of nuclear medicine images include bone and whole body imaging. A very promising capability of the technology is the improved attenuation correction in cardiac imaging. Because of the improved signal quantification the unit produces through more accurate attenuation correction and image segmentation approaches, it will be of particular value in new research protocols such as new radioimmunotherapy approaches being developed together with investigators in Medical Oncology and Pediatric Oncology at UCSF. This promises to make in vivo dosimetry with nuclear medicine radiopharmaceuticals much more accurate than with more traditional methods. |
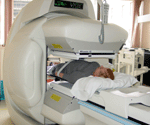 Figure 2: A GE Millenium VH ("Hawkeye") SPECT/CT system recently was installed in the Nuclear Medicine Program within the Department of Radiology at UCSF. This system combines SPECT, coincidence imaging of FDG, and low-resolution CT in a single integrated system, and now is used for clinical imaging at UCSF.
Figure 2: A GE Millenium VH ("Hawkeye") SPECT/CT system recently was installed in the Nuclear Medicine Program within the Department of Radiology at UCSF. This system combines SPECT, coincidence imaging of FDG, and low-resolution CT in a single integrated system, and now is used for clinical imaging at UCSF.
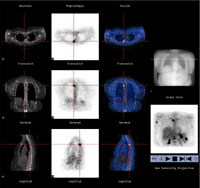 Figure 3: GE Millenium VH (see Figure 2) produces images that allow correlation of functional information from SPECT or FDG with anatomical information from CT. Illustrated above are images from a patient with metastatic melanoma. There is a focus of increased FDG (F-18 fluorodeoxyglucose) uptake in pedicle of T4 consistent with metastatic focus. Normally, the Hawkeye system is used to image single photon emitting radiopharmaceuticals, but the unit installed at UCSF has a thick crystal and coincidence circuitry, making it possible to image FDG and other positron emitting compounds as shown here. Images shown are CT on left ("Anatomic"), FDG in next row ("Physiologic"), and fused (combined) CT and FDG images in the next row, with a scout CT image on the right. A three-dimensional FDG projection image (lower right) shows additional metastases in the right humerus and multiple metastases in the spleen.
Figure 3: GE Millenium VH (see Figure 2) produces images that allow correlation of functional information from SPECT or FDG with anatomical information from CT. Illustrated above are images from a patient with metastatic melanoma. There is a focus of increased FDG (F-18 fluorodeoxyglucose) uptake in pedicle of T4 consistent with metastatic focus. Normally, the Hawkeye system is used to image single photon emitting radiopharmaceuticals, but the unit installed at UCSF has a thick crystal and coincidence circuitry, making it possible to image FDG and other positron emitting compounds as shown here. Images shown are CT on left ("Anatomic"), FDG in next row ("Physiologic"), and fused (combined) CT and FDG images in the next row, with a scout CT image on the right. A three-dimensional FDG projection image (lower right) shows additional metastases in the right humerus and multiple metastases in the spleen.
Optimizing PET-CT Quantification to Radiation Treatment Planning in Treating Cancers of the Head and Neck
Written in 2008
- Nick G. Costouros, MD
- Youngho Seo, PhD
- Stephen L. Bacharach, PhD
- Benjamin L. Franc, MD
- Randall A. Hawkins, MD, PhD
- Henry F. VanBrocklin, PhD
- Bruce H. Hasegawa, PhD
Medical imaging plays a critical role in identifying the size and extent of tumors in patients with cancer, and now has an important role in planning strategies to treat cancer with surgery and radiation therapy. Traditionally, cancer imaging has relied on structural imaging methods such as computed tomography (CT) and magnetic resonance imaging (MRI). These identify the anatomical extent and location of the cancer, typically using intravenously administered contrast agents to identify regions of tumor enhancement. However, CT and MRI are not definitive, in that normal tissue can enhance following surgery and other interventions; nor do they reveal cancer at early stages before anatomical and structural changes become apparent.
 Today, radionuclide imaging using positron emission tomography (PET) is used to identify the uptake and distribution of the radiopharmaceutical 18F-fluorodeoxygluocse(FDG) to identify metabolically active sites, including cancer, in the body. FDG has high uptake in metabolically active areas and can reveal cancer at an earlier stage than standard anatomical methods. However, interpreting FDG PET images can be complicated by its uptake in normal tissues with high metabolic activity, its excretion by the kidneys into the urinary tract, and by high uptake in areas of inflammation that arise following surgery and other interventions. Given these limitations and the potential for false positive increased metabolism on FDG PET, we are investigating a new PET radiopharmaceutical imaging agent, 18F-fluorothymidine (FLT), that is taken up in proportion to cellular proliferation. FLT offers a new signature for cancer imaging with PET, one that can complement the identification of metabolically active areas imaged with FDG PET. In addition, FLT is believed to be less sensitive to inflammation and likely can be used sooner than FDG in studies following therapy to identify recurrent or residual cancer.
Today, radionuclide imaging using positron emission tomography (PET) is used to identify the uptake and distribution of the radiopharmaceutical 18F-fluorodeoxygluocse(FDG) to identify metabolically active sites, including cancer, in the body. FDG has high uptake in metabolically active areas and can reveal cancer at an earlier stage than standard anatomical methods. However, interpreting FDG PET images can be complicated by its uptake in normal tissues with high metabolic activity, its excretion by the kidneys into the urinary tract, and by high uptake in areas of inflammation that arise following surgery and other interventions. Given these limitations and the potential for false positive increased metabolism on FDG PET, we are investigating a new PET radiopharmaceutical imaging agent, 18F-fluorothymidine (FLT), that is taken up in proportion to cellular proliferation. FLT offers a new signature for cancer imaging with PET, one that can complement the identification of metabolically active areas imaged with FDG PET. In addition, FLT is believed to be less sensitive to inflammation and likely can be used sooner than FDG in studies following therapy to identify recurrent or residual cancer.
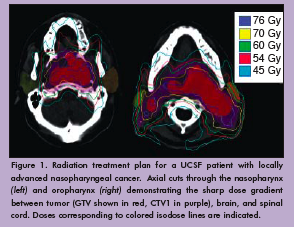
Nearly 60,000 patients are diagnosed with head and neck cancer every year in the United States. The majority will be treated with radiation therapy, either alone or in combination with chemotherapy, surgery, or both. Intensity-modulated radiotherapy (IMRT) is an advanced form of radiation treatment that uses non-uniform radiation beams and computer optimization to distribute radiation doses that conform extremely well to the tumor volume. Using IMRT, the radiation oncologist also can avoid delivering high levels of radiation to nearby critical structures such as the brain stem, spinal cord, and parotid glands (Figure 1). Our project has three goals, to implement:
- novel image correction algorithms
- measures of biologic activity derived from PET-CT
- display formats for improving the use of biologic tumor volumes in planning IMRT of head and neck cancer
Methods
|
We measured the direct correlation of FDG and FLT uptake within primary tumors and lymph nodes and correlated them against tumor presence, Ki-67 index (positive cells/total cells), and the nuclear-to-cytoplasmic ratio using data from post-surgical pathology. These correlations are intended to relate the biological activity and the probability of tumor involvement to measured values of FDG and FLT uptake in the tumor and lymph node. Probability data generated from the PET-CT data will be used to draw theoretical radiation treatment plans for comparison against the formal treatment planning method. |
Results
|
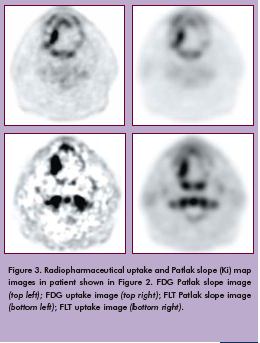
To date, our team has used FDG and FLT with PET-CT to evaluate three patients (Figure 2). All patients tolerated the protocol well. Figure 3 shows FDG and FLT PET images in one patient by both standard uptake methods and by Patlak analysis. Corresponding pathology in this patient demonstrated an increased Ki-67 index in both the primary tumor and reactive lymph nodes (Figure 4). Primary tumor, metastatic lymph nodes, and reactive lymph nodes all showed increased FDG and FLT uptake (Figure 5). Additional data are needed to determine probability and proliferation thresholds for application to radiation treatment planning. However, given the overlap in uptake values between tumor and reactive lymph nodes, this finding underscores the importance of determining thresholds based on pathologic correlation to improve the accuracy of radiation treatment planning volumes.
|
Conclusion
|
The overall project aims to address a fundamental limitation of current quantitative measures of radiopharmaceutical localization on PET-CT that leads to potentially significant variability in cancer therapies. In considering the integration of biologic tumor volumes determined by PET-CT into a radiation treatment plan for patients with cancers of the head and neck, there is potentially a significant inter- and intra-user variability in the implementation of PET-CT in the treatment plan. This is due to a combination of inaccurate quantitative PET-CT data, the current disconnect between quantitative PET-CT data and biologically relevant information, and the inability to display data regarding multiple biological markers along with anatomical data from CT on a single interface for the radiation oncologist. The value that biologic tumor volumes could contribute to the field of radiation oncology is promising, but could be rapidly discounted if arbitrary methods of inaccurate reconstruction and radiopharmaceutical localization persist. Making quantitatively accurate and biologically meaningful measurements of certain biologic characteristics with PET on a voxel-by-voxel basis, overlaid on a precise anatomic map using PET-CT could produce significant advances in the use of biologically active tumor volumes when planning radiation treatment. This has the potential to translate into decreased morbidity and/or mortality. Additional data are still needed to further define these biologically meaningful measurements.
Acknowledgment: NIBIB T32 Training Grant T32 EB001631 (NGC); UC Discovery Grant digit l04-10174 with corporate sponsorship from Siemens Medical Solutions (SLB, BLF, YS); NCI Grant K25 CA114254 (YS)
Stephen L. Bacharach, PhD, is an adjunct professor of Radiology. Nick G. Costouros, MD, is a clinical fellow in the Nuclear Medicine section. Benjamin L. Franc, MD, is a nuclear medicine physician with Radiological Associates of Sacramento Medical Group. Bruce H. Hasegawa, PhD, was director of the Physics Research Laboratory. Randall A. Hawkins, MD, PhD, is a professor of Radiology and chief of the Nuclear Medicine section. Youngho Seo, PhD, is an assistant adjunct professor. Henry F. VanBrocklin, PhD, is a professor in residence and director of Radiopharmaceutical Research at the Center for Molecular and Functional Imaging. |

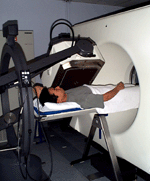 Figure 1: A prototype CT/SPECT system was configured at the UCSF Physics Research Laboratory within the Department of Radiology. It combined a GE 600 XR/T SPECT system with a GE 9800 Quick CT scanner for correlated anatomical and functional imaging.
Figure 1: A prototype CT/SPECT system was configured at the UCSF Physics Research Laboratory within the Department of Radiology. It combined a GE 600 XR/T SPECT system with a GE 9800 Quick CT scanner for correlated anatomical and functional imaging.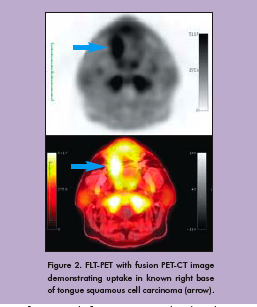 We prospectively enrolled patients with known head and neck cancer who are eligible for tumor and lymph node resection for sequential two-day pre-operative PET-CT imaging with 18F-fluorodeoxyglucose (FDG) and 18F-fluorothymidine (FLT). The patient’s position was repeated by the use of a thermoplastic mask with the neck supported on a Timo cushion mounted on a carbon fiber board. We acquired images in list-mode using a Siemens Biograph 16 PET-CT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) and used software provided by the vendor to reframe them as two dynamic series: 0-10 minutes post-injection over the heart and 15-60 minutes post-injection over the head and neck. A whole body PETCT scan was then performed, followed by a high-count head and neck PET-CT image. We calculated maximum standardized uptake values (SUVs) over specific regions of interest (TrueD software on a syngoMMWP system, Siemens Medical Solutions USA, Inc.). For Patlak graphical analysis, we performed all reconstructions using the same FORE+2D-OSEM algorithm provided by the scannermanufacturer with four iterations and eight subsets. We generated an arterial input function using a left-ventricular region, derived-time activity curve for 0-10 minutes post-injection and measured activity from five venous blood samples thereafter up to one hour post-injection. In the case of FLT, the FLT metabolite fraction was corrected using solid phase extraction and separation. This input function was used to perform Patlak analysis with the dynamic head and neck dataset of 15-60 minutes post-injection. Patlak Ki (slope) images were generated using OASIS, IDL-based tools developed at the National Institutes of Health, Bethesda, MD.
We prospectively enrolled patients with known head and neck cancer who are eligible for tumor and lymph node resection for sequential two-day pre-operative PET-CT imaging with 18F-fluorodeoxyglucose (FDG) and 18F-fluorothymidine (FLT). The patient’s position was repeated by the use of a thermoplastic mask with the neck supported on a Timo cushion mounted on a carbon fiber board. We acquired images in list-mode using a Siemens Biograph 16 PET-CT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) and used software provided by the vendor to reframe them as two dynamic series: 0-10 minutes post-injection over the heart and 15-60 minutes post-injection over the head and neck. A whole body PETCT scan was then performed, followed by a high-count head and neck PET-CT image. We calculated maximum standardized uptake values (SUVs) over specific regions of interest (TrueD software on a syngoMMWP system, Siemens Medical Solutions USA, Inc.). For Patlak graphical analysis, we performed all reconstructions using the same FORE+2D-OSEM algorithm provided by the scannermanufacturer with four iterations and eight subsets. We generated an arterial input function using a left-ventricular region, derived-time activity curve for 0-10 minutes post-injection and measured activity from five venous blood samples thereafter up to one hour post-injection. In the case of FLT, the FLT metabolite fraction was corrected using solid phase extraction and separation. This input function was used to perform Patlak analysis with the dynamic head and neck dataset of 15-60 minutes post-injection. Patlak Ki (slope) images were generated using OASIS, IDL-based tools developed at the National Institutes of Health, Bethesda, MD.