Wilson Lab
The goal of research in the Wilson lab is to develop metabolism and microenvironment-targeted imaging methods, to guide intervention and predict response to molecular therapies. We are currently developing cutting-edge positron emission tomography (PET) and magnetic resonance imaging (MRI) techniques to study basic biochemical pathways and their alterations in infection, cancer and other disorders. In particular, the group pursues imaging targets relevant to the central nervous system and the practice of clinical neuroradiology. New chemistries are used in the design and synthesis of imaging probes, which have strong potential for translation into the clinic. Several tracers developed in the lab are currently being investigated in UCSF patients. We are particularly focused on the molecular imaging of infection using bacteria-specific metabolic radiotracers and MR-compatible methods employing stable isotopes.
News
Comings and Goings
- Congratulations to Dr. Matthew Parker who received a faculty position at Stony Brook University.
- Congratulations to Dr. Jaehoon Shin who is starting a faculty position in the Department of Radiology and Biomedical Imaging July 2022.
- Congratulations to Aryn Alanizi, UCSF Master of Science in Biomedical Imaging (MSBI) graduate who will be starting medical school at Eastern Virginia Medical School in summer 2022.
- Congratulations to Dr. Chao Wang who accepted a patent law position in San Diego.
- Congratulations to Dr. Hecong Qin who will be starting medical school at the University of Chicago summer 2022.
- Congratulations to Dr. Ilona Polvoy who will be continuing her internal medicine residency in Israel.
- Welcome Sarah Rabbitt (2021) who is a synthetic chemist and recent graduate of the University of San Francisco.
- Welcome Sanghee Lee (2022) who is a chemist/radiochemist to received his Ph.D. from Seoul National University.
- Welcome Marina Alvarez Lopez (2022) who is a microbiologist who received her Ph.D. form the University of Groningen.
- Welcome Ryan Nillo (2022) who is a staff research associate having worked previously with Drs. Sugrue and Desikan at UCSF.
- Welcome Priamo Pichardo Gonzalez (2022) who is a medical student from Puerto Rico and future diagnostic radiology resident.
Manuscripts
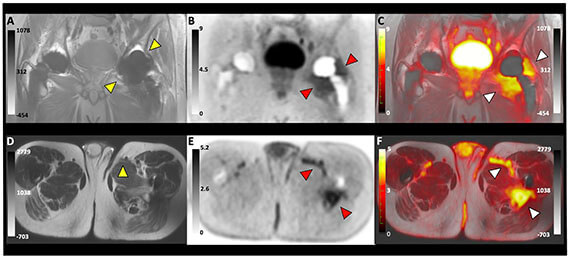
- Dr. Polvoy’s manuscript, “Imaging joint infections using D-methyl-11C methionine PET/MRI: initial experience in humans” published in EJNMMI (2022). https://pubmed.ncbi.nlm.nih.gov/35732972/
- Dr. Polvoy’s manuscript, “Nuclear imaging of bacterial infection: state of the art and future directions” published in JNM (2020). https://jnm.snmjournals.org/content/61/12/1708
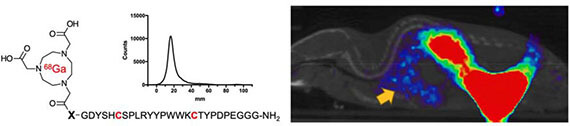
- Dr. Parker’s manuscript, “Cyclic 68Ga- labeled peptides for specific detection of human angiotensin-converting enzyme 2” published in JNM (2021). https://pubmed.ncbi.nlm.nih.gov/33637588/
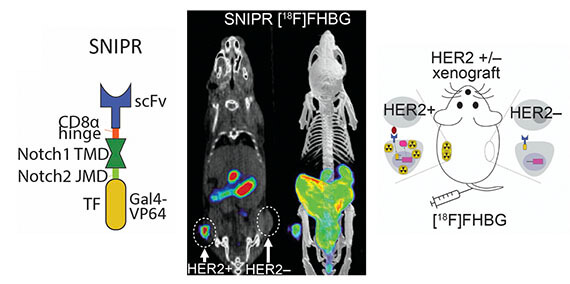
- Dr. Jaehoon Shin’s manuscript, “Antigen-dependent inducible T cell reporter system for PET imaging of breast cancer and glioblastoma” accepted at JNM; preprint @ bioRxiv (2022). https://www.biorxiv.org/content/10.1101/2022.02.16.480729v1
- Dr. Jianbo Liu’s manuscript, “Synthesis of N-trifluoromethyl amides from carboxylic acids” published at Chem (2021). https://www.cell.com/chem/pdf/S2451-9294(21)00361-2.pdf
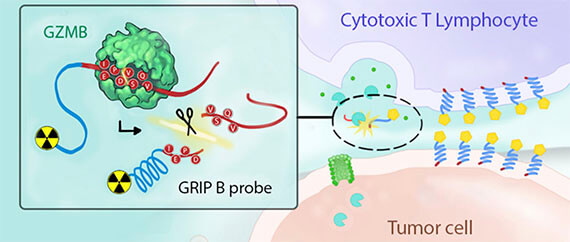
- Dr. Zhao’s manuscript, “In vivo measurement of granzyme proteolysis from activated immune cells with PET” published in ACS Central Science (2021). Collaboration with Evans, Craik labs. https://pubs.acs.org/doi/full/10.1021/acscentsci.1c00529
- Congratulations to Drs. Shin, Polvoy, Qin, Sorlin, Parker, and Alanizi for scientific abstracts accepted at WMIC, Pacifichem, ISRS, and EMIM.
Awards
- R21 “Imaging bacterial infection using deuterium-enriched sugar alcohols"(PI Wilson). 2021.
- R01 “First-in-human evaluation of an astrocytic glutamate transporter (EAAT2) PET tracer in healthy and Alzheimer’s diseased brain" (PIs Wilson, VanBrocklin). 2022.
- R01 “Sensing living P. Aeruginosa using D-alanine derived radiotracers” (PIs Wilson, Ohliger, Engel). 2021.
- R01 “New radiotracer development to study immune cell mobilization of granzyme proteolytic activity” (PIs Evans, Wilson, Craik). 2021.
- R21 “Diagnosis of low-grade glial tumors using 11C glutamine isotopomers” (PIs Villanueva-Meyer, Wilson). 2021.
- R01 “ACE2-targeted PET radiotracers for investigating the spatiotemporal distribution of SARS-CoV-2 organ injury and therapy response” (PIs Wilson, Flavell, Desai). 2021.
- UCSF resource allocation program (RAP) grant for 18F infection imaging translational expenses. 2022.
- R01 “Imaging cerebral metabolic impairment in AD using deuterium MRI” (PIs Chaumeil, Gordon, Wilson). 2022.
- Cystic Fibrosis Foundation (CFF) “Mechanisms of P. Aeruginosa colonization in cystic fibrosis” (PIs Wilson, Engel). 2021.
Opportunities
Publications
Featured Publications
- Shin J, Parker MFL, Zhu I, Alanizi A, Rodriguez CI, Liu R, Watchmaker PB, Kalita M, Blecha J, Liu J, Wright B, Lapi SE, Flavell RR, Okada H, Tlsty TD*, Roybal T*, Wilson DM*. Antigen-dependent inducible T cell reporter system for PET imaging of breast cancer and glioblastoma (2022).Accepted at JNM; pre-print posted on BioRxiv.
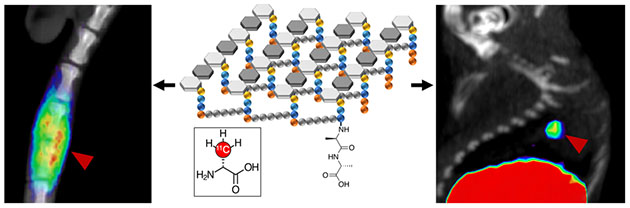
- Polvoy P, Seo Y, Parker M, Stewart M, Siddiqua K, Manasca HS, Ravanfar V, Blecha J, Hope TA, Vanbrocklin H, Flavell R, Barry J, Hansen E, Villanueva-Neyer J, Engel J, Rosenberg OS, Wilson DM*, Ohliger MA*. Imaging joint infections using D-methyl-11C methionine PET/MRI: initial experience in humans (2022). EJNMMI
- Parker MFL, Blecha B, Rosenberg O, Ohliger M, Flavell RR, Wilson DM*. Cyclic 68Ga-labeled peptides for specific detection of human angiotensin-converting enzyme 2 (2021). JNM
- Liu J, Parker MFL, Wang S, Flavell RR, Toste FD*, Wilson DM*. Synthesis of N-trifluoromethyl amides from carboxylic acids (2021). Chem

-
Zhao N, Bardine C, Lourenco AL, Wang Y, Huang Y, Cleary SJ, Wilson DM, Oh DY, Fong L, Looney MR, Evans MJ*, Craik CS*. In vivo measurement of granzyme proteolysis from activated immune cells with PET (2021). ACS Central Science
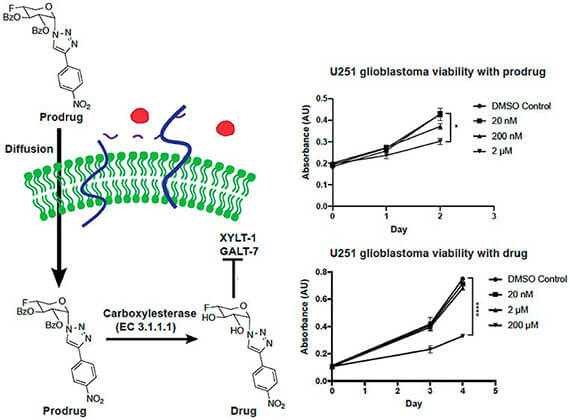
- Kalita M, Villanueva-Meyer J, Ohkawa Y. Kalyanaraman C, Chen K, Mohamed E, Parker MFL, Jacobson MP, Phillips JJ, Evans MJ*, Wilson DM*. Synthesis and screening of a-xylosides in human glioblastoma cells (2020). Molecular Pharmaceutics, epub ahead of print.
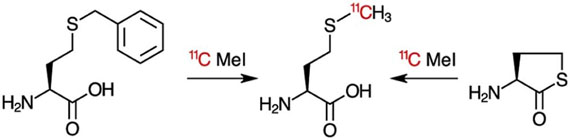
- Parker MFL, Blecha JE, Schulte B, Luu JM, Stewart MN, Flavell RR, VanBrocklin HF, Rosenberg OS, Ohliger MA, Wilson DM. Automated synthesis and uptake analysis of D-[methyl-11C]methionine (2020). Journal of Visualized Experiments (JOVE).
![Automated synthesis and uptake analysis of D-[methyl-11C]methionine](/sites/default/files/wysiwyg/research/wilson/blocking-D11Cmethionine-across-3-pathogens.jpg)
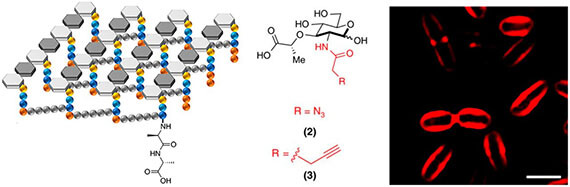
- Parker MFL, Flavell R, Liu J, Rosenberg O, Ohliger M, Wilson DM*. Small molecule sensors targeting the bacterial cell wall (2020). ACS Infectious Diseases 6(7): 1587-1598.
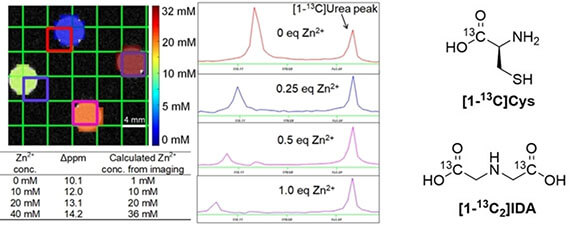
- Wang S, Korenchan DE, Perez PM, Taglang C, Hayes TR, Sriram R, Bok R, Hong AS, Wu Y, Li H, Wang Z, Kurhanewicz J, Wilson DM, Flavell RR*. Amino acid-derived sensors for specific Zn2+ detection using hyperpolarized 13C magnetic resonance spectroscopy (2019). Chemistry. 25(51): 11842-11846. (Collaboration with Flavell lab)
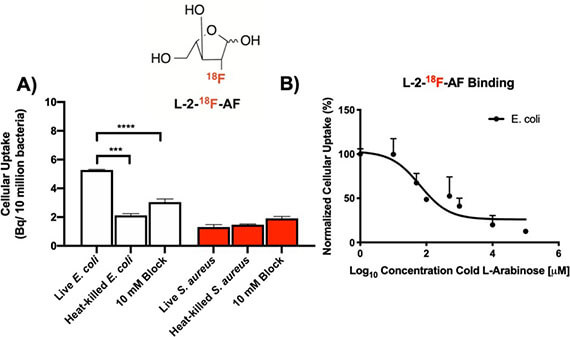
- Kalita M, Parker M, Luu J, Stewart M, Blecha J, VanBrocklin H, Evans M, Flavell R, Rosenberg O*, Ohliger M*, Wilson DM*. Arabinofuranose-derived PET radiotracers for detection of pathologic microorganisms (2020). Journal of Labelled Compounds and Radiopharmaceuticals 63(5): 231-239.
- Stewart MN, Parker MFL, Jivan S, Luu J, Huynh T, Schulte B, Seo Y, Blecha JE, Villanueva-Meyer J, VanBrocklin H, Flavell R, Ohliger M*, Rosenberg O*, Wilson DM*. High enantiomeric excess in-loop synthesis of d-[methyl-11C]methionine for use as a diagnostic positron emission tomography radiotracer in bacterial infection (2020). ACS Infectious DIseases 6(1): 43-49.
![High enantiomeric excess in-loop synthesis of d-[methyl-11C]methionine for use as a diagnostic positron emission tomography radiotracer in bacterial infection](/sites/default/files/wysiwyg/research/wilson/id9b00196_0005.jpg)
- Qin H, Zhang V, Bob R, Delos Santos R, Cunha A, Hsu I, Delos Santos J, Lee J, Subramaniam S, Larson P, Vigneron D, Wilson DM* , Sriram R*, Kurhanewicz K*. Simultaneous metabolic and perfusion imaging using hyper polarized 13C MRI can evaluate early and dose-dependent responses to prostate cancer radiotherapy (2020). The International Journal of Radiation Oncology, Biology, and Physics 107(5): 887-896.
- Polvoy I, Flavell R, Rosenberg O*, Ohliger M*, Wilson DM*. Nuclear imaging of bacterial infection- the state of the art and future directions (2020). The Journal of Nuclear Medicine 61(12): 1708-1716.
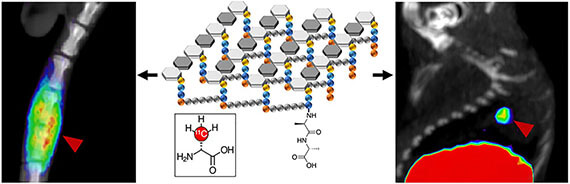
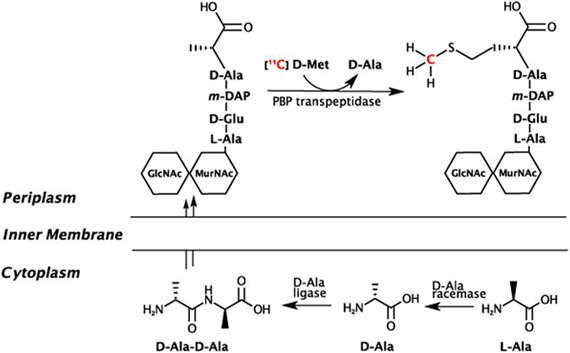
- Parker M, Liu J, Schultz B, Huynh T, Stewart M, Sriram R, Lu M, Jivan S, Turnbaugh P, Flavell R, Rosenberg OS*, Ohliger M*, Wilson DM*. Sensing living bacteria in vivo using D-alanine derived 11C radiotracers (2020). ACS Central Science 6(2): 155-165.
- Dumont RA, Keen N, Bloomer CW, Schwartz BS, Talbott J, Clark AJ, Wilson DM*, Chin CT*. Clinical utility of diffusion-weighted imaging in spinal infections (2019). Clinical Neuroradiology 29(3): 515-522.
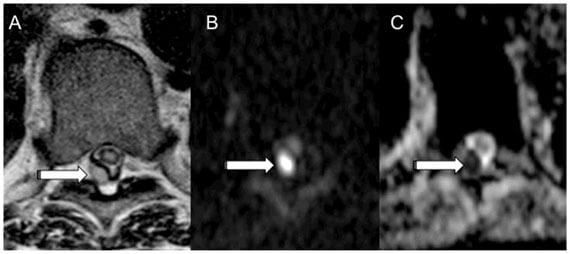
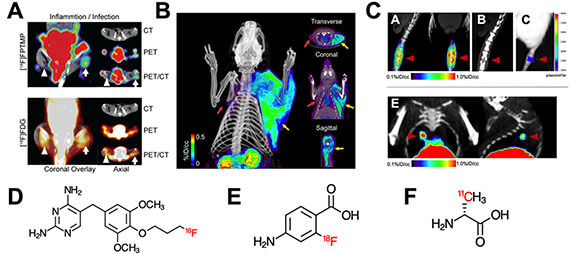
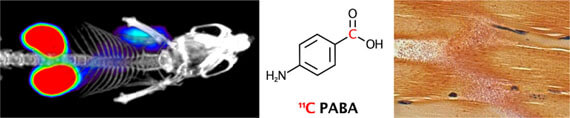
- Mutch CA, Ordonez AA, Villanueva-Meyer JE, Blecha J, Carroll V, Taglang C, Flavell RF, Sriram R, VanBrocklin H, Rosenberg O, Ohliger MA, , Jain SK*, Neumann KD*, Wilson DM*. [11C]Para-aminobenzoic acid: a positron emission tomography tracer targeting bacteria-specific metabolism (2018). ACS Infectious Diseases 4(7): 1067-1072.
- Sriram R, Sun J, Villanueva-Meyer J, Mutch C, De Los Santos J, Peters J, Korenchan DE, Neumann K, Van Criekinge M, Kurhanewicz J, Rosenberg O*, Wilson DM*, and Michael Ohliger*. Detection of bacteria-specific metabolism using hyperpolarized [2-13C] pyruvate (2018). ACS Infectious Diseases 4(5): 797-805.
![Detection of bacteria-specific metabolism using hyperpolarized [2-13C] pyruvate](/sites/default/files/wysiwyg/research/wilson/id-2017-002342_0006.jpg)
- Taglang C, Korenchan K, Von Morze C, Yu J, Najac C, Wang S, Blecha J, Subramaniam S, Bok R, VanBrocklin H, VIgneron D, Ronen S, Sriram R, Kurhanewicz J, Wilson DM, Flavell R*. Late-stage deuteration of 13C-enriched substrates for T1 prolongation in Hyperpolarized 13C MRI (2018). Chemical Communications 54(41): 5233-5236. (Collaboration with Flavell lab)
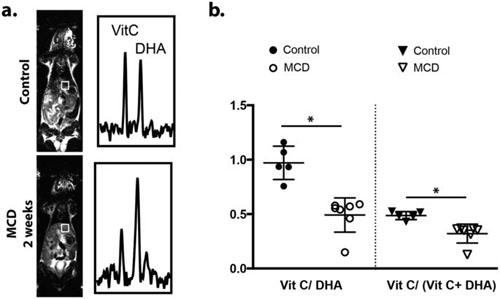
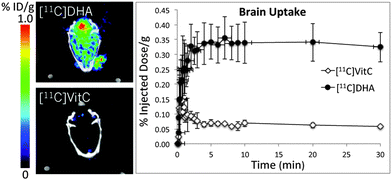
- Qin H, Carroll VN, Sriram R, Villanueva-Meyer JE, von Morze C, Wang ZJ, Mutch CA, Keshari KR, Flavell RR, Kurhanewicz J, Wilson DM*. Imaging glutathione depletion in the rat brain using ascorbate-derived hyperpolarized MR and PET probes (2018). Scientific Reports 8(1): 7928.
-
Neuman K, Villanueva-Meyer J, Mutch C, Blecha J, VanBrocklin H, Rosenberg O*, Ohliger M*, Wilson DM*. Imaging Active Infection in vivo Using D-Amino Acid Derived PET Radiotracers (2017). Scientific Reports 7(1): 7903.
-
Neuman K, Flavell RF, Wilson DM*. Exploring metabolism in vivo using endogenous 11C metabolic tracers (2017). Seminars in Nuclear Medicine 47(5):461-473.
-
Wilson DM, Di Gialleonardo V, Wang ZJ, Taylor A, Sai V, VanCriekinge M, Bok R, Ohliger MA, Keshari KR*. Imaging oxidative stress in non-alcoholic fatty liver disease using hyperpolarized 13C MRI. Scientific Reports 2017 (epub ahead of print).
-
Liu W, Truillet C, Flavell RR, Brewer T, Evans MJ, Wilson DM*, Chang C*. A reactivity-based [18F]FDG probe for in vivo formaldehyde using positron emission tomography. Chemical Science 2016; 7: 5503-07.
![A reactivity-based [18F]FDG probe for in vivo formaldehyde using positron emission tomography](/sites/default/files/wysiwyg/research/wilson/Get.jpeg)
-
Carroll V, Truillet C, Shen B, Flavell RF, Shao X, Evans MJ, Vanbrocklin HF, Scott PJ*, Chin FT*, Wilson DM*. [11C]Ascorbic and [11C]dehydroascorbic acid, an endogenous redox pair for sensing reactive oxygen species using positron emission tomography. Chemical Communications 2016; 52: 4888-4890.
-
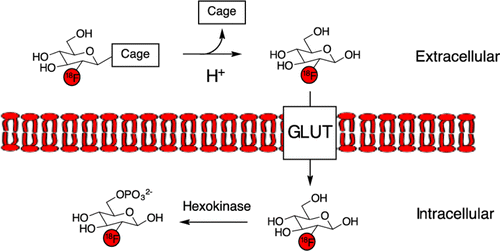
Flavell RR, Truillet C, Regan MK, Gangly T, Blecha JE, Kurhanewicz J, Vanbrocklin HF, Keshari KR, Chang CJ, Evans MJ, Wilson DM*. Caged 18F-glycosylamines for imaging acidic tumoral microenvironment using positron emission tomography. Bioconjugate Chemistry 2016; 27(1): 170-178.
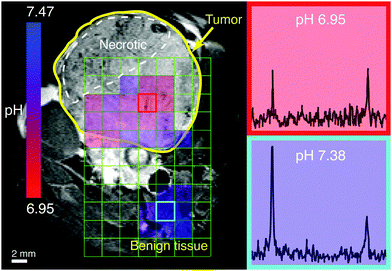
- Korenchan DW, Flavell RR, Baligand C, Sriram R, Neumann K, Subramaniam S, Vanbrocklin HF, Vigneron DB, Wilson DM*, Kurhanewicz J*. Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging. Chemical Communications 2016; 52(14): 3030-3033.
-
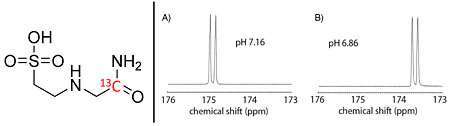
Flavell RR, Von Morze C, Blecha JE, Korenchan DE, Van Cricking M, Sriram R, Gordon JW, Chen HY, Subramaniam S, Bok RA, Wang ZJ, Vigneron DB, Larson PE, Kurhanewicz J, Wilson DM*. Application of Good's buffers to imaging using hyperpolarized 13C MRI. Chemical Communications 2015; 51(74): 14119-14122.
-
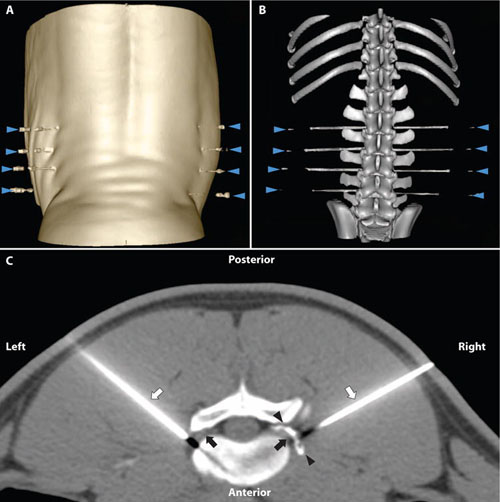
Brown J, Saeed M, Braz J, Basbaum AI, Iadarola MJ, Wilson DM, Dillon B*. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Science Translational Medicine 2015; 7(305): 305ra145.39.
-
Keshari K, Wilson DM, VanCriekinge M, Sriram R, Koelsch B, Wang Z, VanBrocklin H, Peehl D, Kurhanewicz J*. Metabolic response of prostate cancer to nicotinamide phosphoribosyl transferase inhibition in a hyperpolarized MR/PET compatible bioreactor. Prostate 2014; 75(14): 1601-1609.
-
Keshari K, Wilson DM, Sai V, Bok R, Jen K, Larson P, VanCriekinge M, Kurhanewicz J, Wang Z*. Non-invasive in vivo imaging of diabetes-induced renal oxidative stress and response to therapy using hyperpolarized 13C dehydroascorbate magnetic resonance. Diabetes 2015; 64: 344-352.
-
Carroll V, Michel B, Blecha J, VanBrocklin H, Keshari K, Wilson DM*, Chang C*. A boronate-caged 18F-FLT probe for hydrogen peroxide detection using positron emission tomography. Journal of the American Chemical Society 2014; 136(42):14742-14745.
-
Keshari KR, Wilson DM*. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chemical Society Reviews 2014; 43: 1627-1659.
-
Keshari KR, Sai V, Wang ZJ, VanBrocklin HF, Kurhanewicz J, Wilson DM*. Hyperpolarized 13C MR spectroscopy using [1-13C] dehydroascorbate in a murine model of prostate cancer: comparison with 18F-FDG. J Nuc Med. 2013; 54(6): 922-928.
![Hyperpolarized 13C MR spectroscopy using [1-13C] dehydroascorbate in a murine model of prostate cancer: comparison with 18F-FDG](/sites/default/files/wysiwyg/research/wilson/pub7.jpg)
- Wilson DM*, Bergauer M, Perlson L, Keshari KR. Solid phase synthesis of hydroxamate peptides for histone deacetylase inhibition. Tet Letters 2013; 54: 151-153.
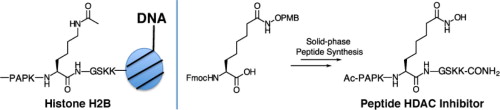
-
Keshari KR, Sriram R, Koelsch BL, Van Criekinge M, Wilson DM, Kurhanewicz J, Wang ZJ*. Hyperpolarized 13C pyruvate MR reveals rapid lactate export in metastatic human renal cell carcinoma cells. Cancer Res. 2012; 73: 529-538.
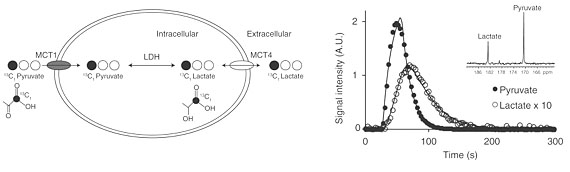
-
Keshari KR, Kurhanewicz J, Macdonald JM, Wilson DM*. Generating contrast in hyperpolarized 13C MRI using ligand-receptor interactions. Analyst 2011; 137: 3427–3429.
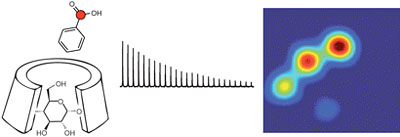
-
Keshari K, Kurhanewicz J, Bok R, Larson P, Vigneron D, Wilson DM*. Hyperpolarized [1-13C] dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proc Nat Acad Sci. 2011; 108: 18606-18611.
![Hyperpolarized [1-13C] dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging](/sites/default/files/wysiwyg/research/wilson/pub4.jpg)
-
Wilson DM, Keshari K, Larson P, Chen A, Hu S, VanCriekinge M, Bok R, Nelson S, Macdonald J, Vigneron D, Kurhanewicz J*. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J Magn Reson. 2010; 205(1): 141-7.
-
Keshari K, Wilson DM, Chen A, Bok R, Larson P, Hu S, VanCriekinge M, Macdonald J, Vigneron D, Kurhanewicz J*. Hyperpolarized [2-13C]-fructose: a hemiketal substrate for in vivo metabolic imaging. J Am Chem Soc. 2009; 131(48): 17591-6.
![Hyperpolarized [2-13C]-fructose: a hemiketal substrate for in vivo metabolic imaging](/sites/default/files/wysiwyg/research/wilson/pub2.jpg)
-
Wilson DM, Hurd R, Keshari K, VanCriekinge M, Chen A, Nelson S, Vigneron D, Kurhanewicz J*. Generation of hyperpolarized substrates by secondary labeling with [1,1-13C] acetic anhydride. Proc Nat Acad Sci. 2009; 106(14):5503-07.
![Generation of hyperpolarized substrates by secondary labeling with [1,1-13C] acetic anhydride](/sites/default/files/wysiwyg/research/wilson/pub1.jpg)
People
 David M. Wilson, MD, PhD
David M. Wilson, MD, PhD
Professor in Residence
[email protected]
David M. Wilson, M.D., Ph.D. received his B.S. degree from Harvard University in Biochemistry, and completed his M.D./Ph.D. training at Columbia University in New York City. His Ph.D. mentor was Dr. Ronald Breslow, a pioneer in artificial enzymes, the hydrophobic effect, and bio-organic chemistry. Dr. Wilson’s subsequent clinical training was in radiology and neuroradiology at University of California, San Francisco (UCSF), where he trained further in the laboratory of Professor John Kurhanewicz and subsequently became a faculty member in 2010. He has since established a basic science laboratory investigating the detection and characterization of cancer via analyte sensing, and developing probes for positron emission tomography (PET) and hyperpolarized 13C spectroscopy. His laboratory has most recently studied imaging infection using bacteria-specific metabolic pathways, and ways to detect ACE2 suppression in SARS-CoV-2.
 Joanne Engel, MD, PhD
Joanne Engel, MD, PhD
Professor
[email protected]
 Michael Ohliger, MD, PhD
Michael Ohliger, MD, PhD
Associate Professor
[email protected]
 Jaehoon Shin, MD, PhD
Jaehoon Shin, MD, PhD
Assistant Professor
[email protected]
 Javier Villanueva-Meyer, MD
Javier Villanueva-Meyer, MD
Assistant Professor
[email protected]
 Marina Lopez Alvarez, PhD
Marina Lopez Alvarez, PhD
Postdoctoral Fellow
[email protected]
 Sanghee Lee, PhD
Sanghee Lee, PhD
Postdoctoral Fellow
[email protected]
 Priamo A. Pichardo-González
Priamo A. Pichardo-González
[email protected]
 Ryan Nillo
Ryan Nillo
SRA
[email protected]
 Sarah Rabbitt
Sarah Rabbitt
SRA
[email protected]
 Alexandre Sorlin, PhD
Alexandre Sorlin, PhD
Postdoctoral Fellow
 Joseph Blecha, MS
Joseph Blecha, MS
Specialist
[email protected]
 Teri Moore
Teri Moore
Lab Manager
[email protected]
Former Lab Members
- Oren Rosenberg, MD, PHD is head of bacterial infections R&D at Johhson and Johnson Pharmaceuticals.
- Ilona Polvoy, MD is an internal medicine resident at Rambam Health Care.
- Chao Wang, PhD is a scientific advisor at Jones Day Law Firm in San Diego.
- Aryn Alanizi, MS is a medical student at East Virginia Medical School.
- Matthew Parker PhD is an Assistant Professor at Stony Brook University.
- Hecong Qin, PhD is a medical student at University of Chicago.
- Christopher Mutch, MD, PhD is a practicing neuroradiologist at Bay Imaging Consultants.
- Mausam Kalita, PhD is a Specialist in the UCSF Department of Radiology and Biomedical Imaging.
- Megan Stewart, PhD is a Senior Manager at Clovis Oncology.
- Justin Luu is a PhD candidate at Emory University.
- Celine Taglang, PhD is a Postdoctoral Scholar at UCSF
- Rebecca Dumont-Walter, MD is a Neuroradiologist at Geneva, Canton of Geneva (Switzerland).
- Valerie Carroll, PhD is a Staff Scientist at EAG Laboratories.
- Kiel Neumann, PhD is a faculty member at the University of Virginia Department of Radiology and Medical Imaging
- Robert Flavell, MD, PhD is a UCSF Radiology and Biomedical Imaging faculty member and close collaborator
- Dongdong Liang, PhD is a chemistry department faculty member in China.
- Jianbo Liu, PhD is a faculty member at Sun Yat-Sen University in China.
- Andrew Taylor, MD, PhD is a UCSF Radiology and Biomedical Imaging faculty member.
- Victor Sai, MD is a UCLA Diagnostic Radiology faculty member.
- Wei Liu, PhD is a faculty member at the University of Cincinnati.
- Tanushree Ganguly, PhD is a Project Scientist at the UCD Department of Biomedical Engineering.
- Jacob Brown, MD, PhD is a neuroradiologist at Utah Radiology Associates.
- J.P. Yu, MD, PhD is a University of Wisconsin Department of Radiology faculty member.
- Kayvan Keshari, PhD is a MSKCC faculty member.
Resources
UCSF
- University of California, San Francisco
- UCSF Department of Radiology
- UCSF Neuroradiology
- UCSF Mission Bay
- QB3
- Campus Life Services (Housing, gym)
- Chemistry and Chemical Biology
- UCB-UCSF Bioengineering
- Small Molecule Discovery Center
Labs
- John Kurhanewicz
- Michael Evans
- Henry VanBrocklin
- Jane Wang
- Michael Ohliger
- Dan Vigneron
- Niren Murthy (UC Berkeley)
- Kayvan Keshari (MSKCC)
- Peter Scott (University of Michigan)
- Frederick Chin (Stanford)
- Sanjay Jain (Johns Hopkins)
Chemistry:
Research Directions
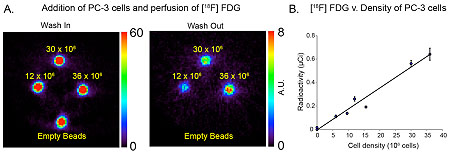
Metabolic imaging of pathogenic microorganisms
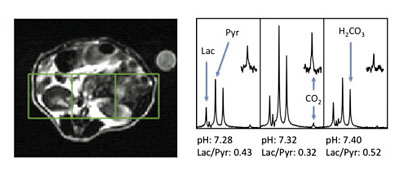
The Wilson laboratory is pursuing clinically translatable biomarkers for infection, particularly discitis-osteomyelitis, which may be difficult to diagnose using computed tomography (CT) and magnetic resonance imaging (MRI). Existing clinical strategies to image bacterial infection in patients rely on activated immune cells (either 111In white blood-cell scan or 18F-FDG) and thus cannot reliably distinguish living bacteria from sterile inflammation. We are studying translational biomarkers for hyperpolarized 13C spectroscopy and positron emission tomography (PET) that target metabolic pathways specific to bacteria. We have developed several agents with excellent clinical promise including D-amino acid derived tracers (collaborative work with co-PI’s Michael Ohliger and Oren Rosenberg). More recently we have developed imaging tools to detect the suppression of ACE2, the primary receptor for SARS-CoV-2 entry, in COVID-19.

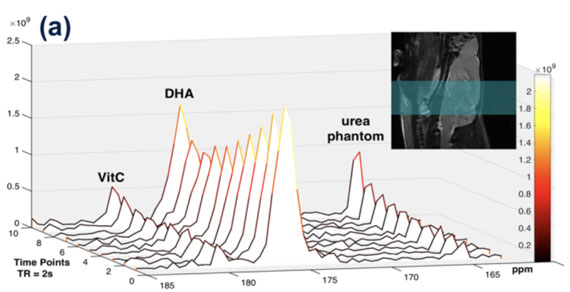
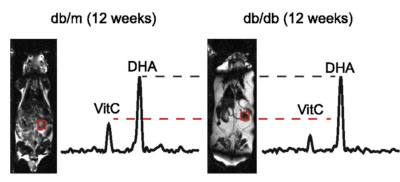
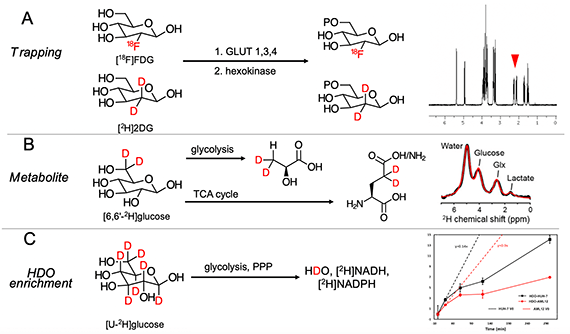
New tools for deuterium metabolic imaging (DMI)
We are investigating new deuterium-enriched probes for the molecular imaging of infection and Alzheimer’s disease (with Drs. Chaumeil, Gordon). The graphic depicts three glucose-derived 2H-enriched molecules we have studied in the normal murine brain and animal models of AD. In collaboration with spectroscopists at UCSF Mission Bay and physicians at the UCSF Memory and Aging Center we are developing tools to use DMI in UCSF patients.
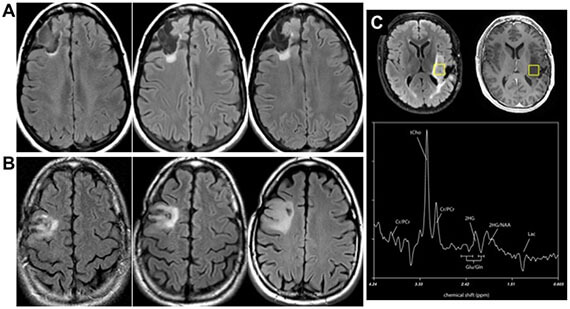
Detection of low-grade glioma and chondrosarcoma via isocitrate dehydrogenase mutants (IDHm)
The Wilson laboratory is developing metabolism-targeted methods to identify tumors harboring IDHm, either (1) based on consumption of tumor-relevant metabolites or (2) direct detection of the IDHm enzyme via modification of known inhibitor structures. These tools will significantly impact the diagnosis and treatment of low-grade glioma and other lesions. These studies are performed in collaboration with the laboratory of Dr. Pavithra Viswanath and clinical collaborator Dr. Javier Villanueva-Meyer.
Developing new chemistry and radiochemistry for positron emission tomography
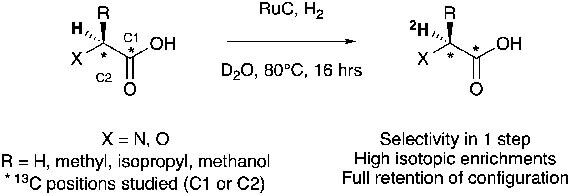
The laboratory is currently developing new ways to incorporate 11C and 18F radionuclei, expanding the arsenal of radiopharmaceuticals applied to biologic problems. We recently developed (Liu et al, Chem 2021) a new method of fluorine incorporation into amide structures that is currently under investigation in our laboratory for 18F labeling of amino-acids and their derivatives.

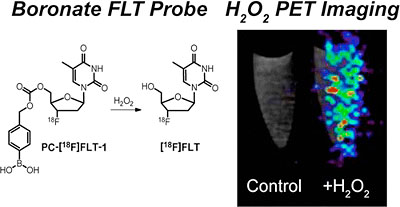
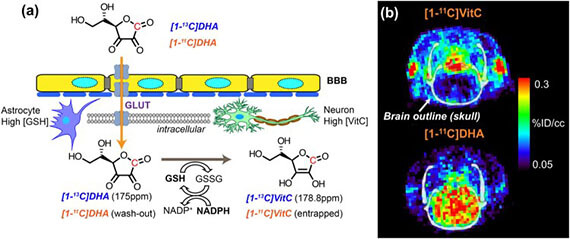
Ascorbate-based sensors for imaging the redox status of tumors using hyperpolarized 13C MR spectroscopy and PET
The redox status of cancer cells is critical in understanding both tumor aggressiveness and response to therapy. In cancer, dysregulation of reactive oxygen species (ROS) is linked to both enhanced tumorgenicity and resistance to common treatments in particular radiotherapy. We are developing new probes for clinically translatable imaging modalities, namely hyperpolarized (HP) 13C MR spectroscopy and positron emission tomography (PET), to study both abundant antioxidant molecules and reactive oxygen species including H2O2. Endogenous reduced glutathione (GSH) concentrations have been explored using hyperpolarized 13C dehydroascorbic acid as a redox sensor. A reaction-based method was employed to study extracellular H2O2 using a boronate-caged prodrug of 18F-FLT. We continue to pursue strategies to study relevant small-molecule analytes in cancer using both endogenous molecules and new chemical strategies.
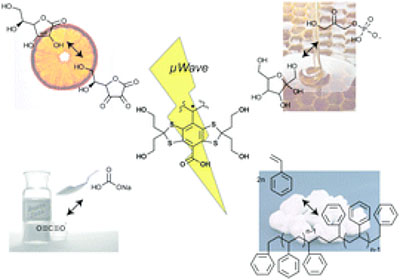
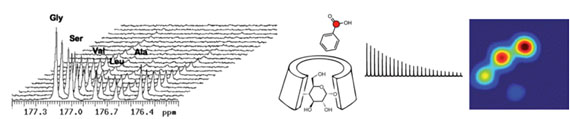
Basic molecular mechanisms in hyperpolarized 13C MRS
The Wilson laboratory is developing hyperpolarized 13C probes that are critical in advancing and understanding dynamic nuclear polarization- nuclear magnetic resonance (DNP-NMR) technology. For example, modification of substrates by hyperpolarized [1,1’-13C2] acetic anhydride demonstrated the transfer of hyperpolarization by chemical means, while study of molecular interactions in solution used a 13C benzoate/ cyclodextrin model system. We are currently investigating hyperpolarized 13C probes with an analyte-dependent chemical shift for studying microenvironments in vivo. The application of hyperpolarized 13C MRS to numerous molecular imaging strategies is being pursued.
Related Content
- Research Directory
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Quantitative Biomarkers & AI in MSK Imaging
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- Vascular Imaging Research Center
- Viswanath Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services
Wilson Lab
185 Berry Street
China Basin Landing
San Francisco, CA 94107
Ph: (415) 353-1668
Fax: (415) 353-8593

David M. Wilson, MD, PhD
Professor in Residence
