Bone Quality Research Lab (Kazakia Lab)
Our main research interest is the characterization of bone quality in disease states affecting the skeleton using both in vivo and ex vivo high resolution imaging techniques. Specifically, we are developing and applying techniques in high resolution peripheral quantitative computed tomography (HR-pQCT), Magnetic Resonance Imaging (MRI), microCT, and Fourier transform infrared (FTIR) spectroscopy for the assessment of bone quality and bone-related physiology. As a part of the Musculoskeletal Quantitative Imaging Research (MQIR) group in the Department of Radiology and Biological Imaging at UCSF, we enjoy a rich, collaborative environment working closely with researchers, clinicians, and students. We are committed to the training and education of postdoctoral fellows, residents, and students (medical, graduate, undergraduate, and high school) interested in pursuing careers in musculoskeletal research. Our research is supported by the NIH and by intramural UCSF funding.
Major Research Projects
Systemic Bone Loss Following Fracture in Humans
Osteoporosis and low bone mass affect over 50 million Americans, and osteoporosis-related fractures will account for over three million broken bones and $25 billion in related costs each year by 2025. Identifying the underlying causes of bone loss are therefore of utmost importance for improving the skeletal health of a rapidly aging population. Epidemiologically, the most reliable predictor of fracture risk is a history of fracture at any skeletal site. The etiology of this relationship is not fully known and depends on many factors (e.g., low bone mass, poor bone quality, risk of falls), but one underappreciated mechanism is that fracture initiates systemic bone loss throughout the skeleton, which increases future fracture risk at all skeletal sites. Our lab has generated multiple preclinical studies characterizing this post-traumatic systemic bone loss response following fracture in mice. However, post-traumatic bone loss following fracture has never been specifically investigated in human subjects. Therefore, the long-term goal of this research is to address this knowledge gap by determining the time course and magnitude of post-fracture bone loss and recovery in human patients and to determine mechanisms driving this response (e.g., systemic inflammation, decreased activity level, mineral utilization), and eventually to evaluate treatments aimed at preserving skeletal health following fracture in older patients.
In the current project, we use standard clinical and cutting-edge high-resolution imaging to characterize the time course and magnitude of systemic bone loss and recovery following humerus fracture in older patients. Based on currently available clinical data, we hypothesizethat post-fracture systemic bone loss: 1) will persist for 6 months or more post-fracture, 2) will have a greater effect on trabecular bone than cortical bone, and 3) will be greater in patients that exhibit increased post-fracture systemic inflammation.
Co-PI Blaine Christiansen, PhD
Funded by NIH/NIA R01 AG078347
Progression and etiology of cortical porosity in diabetic bone disease
Patients with type 2 diabetes (T2D) have an increased risk for fragility fractures despite normal or even elevated bone mineral density (BMD) Recent findings suggest that diabetic bone exhibits abnormalities in bone quality, including elevated cortical porosity. Cortical porosity has deleterious effects on bone strength, and is critical in fracture initiation and propagation, but the origins and temporal evolution of pathological cortical porosity in T2D are unknown. To develop treatments specifically targeted to the prevention or reversal of pathological cortical porosity and associated bone fragility in T2D, we must understand the mechanisms driving development of these cortical pores. The goal of this study is to investigate the underlying biological processes that drive increased cortical porosity in the setting of T2D and to understand the longitudinal evolution of human diabetic bone disease with a special focus on cortical porosity. We are currently conducting a longitudinal study of pore progression in T2D patients, using a novel combined high-resolution peripheral quantitative computed tomography (HR-pQCT) and dynamic contrast enhanced magnetic resonance imaging (DCE MRI) approach. We are developing novel image analysis approaches to characterize pore content and spatial distribution of porosity within the cortex, and are using micro finite element (μFE) analysis to quantify the biomechanical impact of porosity.
Funded by NIH/NIAMS R01 AR069670
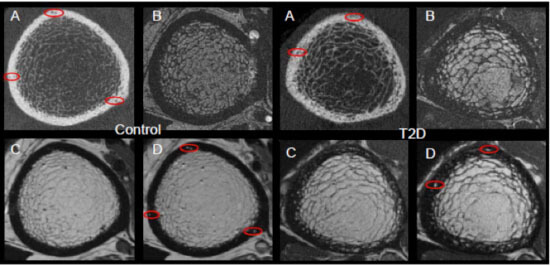
Comparison of (A)HR-pQCT, (B)fat-sensitive MR, (C)pre-contrast MR, and (D)post-contrast MR for a healthy control subject displaying exclusively vessel-filled pores (LEFT) and a T2D subject with both marrow- and vessel-filled pores (RIGHT). Prominent vessels in the post-contrast scans are highlighted in red.
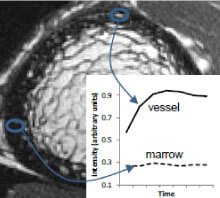
Contrast enhancement curves for regions of hyperintense signal on the post-contrast MR (vessel) and fat-sensitive MR (marrow).
Bone quality and marrow adiposity in HIV-infected individuals
Read about our preliminary results in this UCSF Radiology Blog post!
Low bone mineral density (BMD) and increased fracture risk are increasingly recognized as significant sequelae of HIV infection and treatment. As HIV-infected individuals live longer through effective antiretroviral therapy (ART), HIV-related bone loss is superimposed upon age-related bone loss, resulting in up to 4-fold higher annual rates of fragility fracture in HIV-infected individuals than in the general population. The pathophysiology underlying skeletal changes in the setting of HIV infection appears to differ from that of non-HIV populations experiencing osteoporosis. For example, low BMD does not explain increased fracture risk in HIV-infected individuals. An emerging explanation for this paradox is that HIV infection is associated with bone quality changes – specifically in bone geometry and microstructure – that do not impact BMD but do increase fracture risk. The etiology of these bone quality changes is unknown. Our hypothesis is that, as in non-HIV-infected populations with low BMD, preferential differentiation of adipocytes over osteoblasts results in increased marrow adiposity and associated decreased bone quality.
The aim of this project to apply novel high-resolution image acquisition and analysis to:
1) quantify bone quality deterioration in HIV-infected patients relative to uninfected controls, and
2) determine the relationship between marrow adiposity and bone quality deterioration in HIV-infected patients.
Funded by NIH/NIAID R01 AI125080
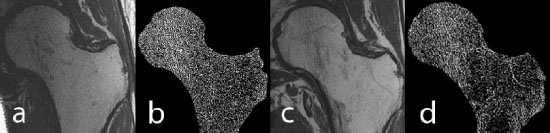
High-resolution MRI of the hip: a) image of a healthy male control, b) trabecular structure after fuzzy thresholding, c) image of an age-matched HIV-infected male subject, d) trabecular structure of the HIV-infected scan. Trabecular structure is less dense in the HIV-infected subject, in particular in the neck, trochanter, and shaft.
Additional Research Directions
HR-pQCT in vivo bone quality assessment
Accurate quantification of bone microarchitecture is significant in understanding bone mechanics and response to disease or treatment. High-resolution peripheral quantitative computed tomography (HR-pQCT) allows for the quantification of trabecular and cortical structure in vivo, with the capability of generating images at multiple resolutions. The aim of this project is to characterize the effect of resolution on structural measures of trabecular and cortical bone and to determine accuracy in reference to micro-CT (µCT), the gold standard for bone microarchitecture quantification.

Figure: Representative images of the trabecular segmentation for both µCT and HR-pQCT images. A) Grayscale images acquired by the respective scanners. B) Trabecular segmentation of a single slice. C) 3D representation of the trabecular volume.
A novel method for quantifying cortical pore topology
Osteonal clustering and merging is increased in hip fracture cases, suggesting that the structural realignment of porosity may be a mechanism of fracture by means of altered local strain and stress fields within cortical tissue. We therefore believe the investigation of pore topology is important to estimating the effects of age, disease, and therapeutic interventions on fracture risk. This project develops a pore topology analysis technique which provides data pertinent to pore clustering, realignment, and interconnectedness. The technique utilizes a skeletonization routine to deconstruct the cortical pore network into individual elements, allowing for the classification of each element as either a plate or a rod. From the skeletonized image, parameters of pore structure and topology can be quantified.

Figure: Pore topology analysis of a tibial cross-section allows for calculation of parameters describing cortical pore structure.
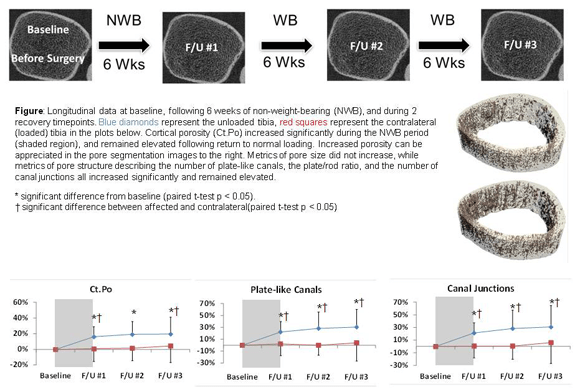
Effects of reduced weight-bearing on skeletal geometry, micro-structure, strength
Significant bone is a common consequence of immobilization or restricted weight-bearing. Previous studies investigating bone loss in this context have focused on changes in bone mineral density (BMD). However, since cortical and trabecular microstructure contribute significantly to bone strength, we quantify and evaluate changes in microstructural and biomechanical parameters at the distal tibia during and after a period of non-weight-bearing. A long-term clinical focus of this research is to facilitate the development of targeted countermeasures to prevent bone loss.
MicroCT quantification of bone composition
The contribution of bonetissuequality to bone mechanics is not captured by bone mineral density or architectural measures. Assessment of bone tissue mineral density (TMD) may be critical to our understanding of bone biomechanics. Although high-resolution 3D assessment of TMD has been performed using synchrotron radiation microcomputed tomography (SRμCT), this imaging modality is not widely available. As conventional desktop μCT systems are far more easily accessed, we compare μCT-based measurements of bone mineral content, TMD, and structure measures to those obtained by SRμCT.

Figure: Comparison of μCT and SR μCT. Top: μCT (left) and SRμCT (right) images of a trabecular bone specimen. Bottom: Histograms of TMD data for a single sample.
Mineralization abnormalities in a mouse model of fibrous dysplasia
It is known that activation of the Gs G-protein coupled receptor (GPCR) pathway in osteoblasts can lead to significant increases in trabecular bone formation. However, the effects of constitutive Gs signaling on bone tissue quality are not known. Using Fourier transform infrared (FTIR) spectroscopy and synchrotron radiation μCT (SRμCT), we evaluate the bone composition of double transgenic mice that exhibit osteoblast-specific constitutive Gs signaling activity. Our findings demonstrate that Gs activity in osteoblasts leads to the deposition of immature bone tissue with reduced mineralization.

Figure: Evaluation of bone quality in double transgenic (DT) compared to wildtype (WT) mice. Top:Full bone renderings (inset) and detailed sections from mid-diaphysis of femurs from 3-week old wildtype (left) and double transgenic (right) mice. Bottom: Mid-diaphyseal cross-section of a 9-week old double transgenic mouse (right) and SRμCT data (left) show a decrease in mineralized bone tissue and a disordered mineralization pattern.
Bone Quality Research Lab
Bone Quality Research Lab Opportunities
Postdoctoral Fellowships
- https://opportunities.ucsf.edu/content/ucsf-postdoctoral-scholar-8
- https://opportunities.ucsf.edu/content/ucsf-postdoctoral-scholar-9
Internships
We have student research assistant positions available for the semester, summer, or for longer term. In particular, we look for highly motivated students with programming experience to help with advanced image processing, or with lab experience to help with cadaver and animal specimen experiments. The students will be trained in all necessary procedures and exposed to both basic science and clinical aspects of musculoskeletal research. Students will also have the opportunity to work closely with radiologists, imaging scientists, and clinicians including orthopaedic surgeons. Potential applicants are encouraged to review the following training programs, in which we actively participate:
Publications
Kazakia Lab publications: https://www.ncbi.nlm.nih.gov/pubmed/?term=Kazakia
People
Current Members
- Sophia Bardenfleth: PhD Student (Visiting)
- Bo Fan: Imaging Specialist
- Celestine Fry: Administrative Assistant
- Lauren Go: Undergraduate Student Researcher (URAP), UC Berkeley
- Katie Kenny: Undergraduate Student Researcher (URAP), UC Berkeley
- Pranav Kolluri: SURF Rose Hills Fellow, UC Berkeley
- Gaik Mkrtchian: Undergraduate Student Researcher (URAP), UC Berkeley
- Stephanie Murphy: Financial Analyst
- Gabby Ramil: MSBI Graduate Student
- Isra Saeed: Senior Clinical Research Coordinator
- Po-hung Wu: Postdoctoral Researcher
- Minhao Zhou: Postdoctoral Researcher
Alumni
- Zehra Akkaya: Fulbright Visiting Scholar
- Gregory Bernstein: Undergraduate Student Researcher (URAP)
- Julio Carballido-Gamio: Senior Scientist
- Karen Cheng: Undergraduate Student Researcher (QUEST/URAP)
- Sam Cheung: Undergraduate Student Researcher (URAP)
- Ashley Chien: Undergraduate Student Researcher (URAP), UC Berkeley
- Sarah Foreman: Postdoctoral Researcher
- Barbara Garita: Research Associate
- Matthew Gibbons: Graduate Student Researcher, UCSF
- Harsh Goel: Undergraduate Student Researcher (URAP)
- Grace Jun: Clinical Research Coordinator
- Rishi Khare: Undergraduate Student Researcher (URAP), UC Berkeley
- Neha Kidambi: Undergraduate Student Researcher (URAP), UC Berkeley
- Xinyu Li: Staff Research Associate
- Maximilian Loeffler: Postdoctoral Fellow
- Cuong Luu: Undergraduate Student Researcher (URAP), UC Berkeley
- Thelma Munoz: Clinical Research Coordinator
- Jasmine Nirody: Graduate Student Researcher (UC Berkeley)
- Joyce Pang: Undergraduate Student Researcher (URAP)
- Robin Parrish: Undergraduate Student Researcher (UC Berkeley)
- Courtney Pasco: Staff Research Associate
- Magdalena Posadzy: Radiologist Fellow
- Amir Pirmoazen: Research Fellow
- Saghi Sadoughi: Postdoctoral Researcher
- Kiranjit Sekhon: Undergraduate Student Researcher (URAP)
- Hsin-Wei Shen: Specialist
- Swetha Shanbhag: Undergraduate Student Researcher (URAP)
- Derek Speer: Undergraduate Student Researcher (URAP)
- Aditya Subramanian: Undergraduate Student Researcher (URAP), UC Berkeley
- Willy Tjong: Staff Research Associate
- Benjamin Ulrich: Undergraduate Student Researcher (URAP), UC Berkeley
- Linda Yang: Undergraduate Student Researcher (URAP), UC Berkeley
- Melis Yilmaz: Undergraduate Student Researcher (URAP), UC Berkeley
- Yang Zhao: Undergraduate Student Researcher





Bone Quality Research Lab Director
- Research Directory
- Abdominal Imaging Research
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- Brain Arteriovenous Malformations
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Daniel B. Vigneron Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Majumdar Lab
- Molecular Imaging Lab (Flavell Lab)
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Magnetic Resonance Imaging Lab (Krug Lab)
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Neuroradiology Research
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Pediatric Research
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- UCSF Nuclear and Clinical Molecular Imaging Research
- Vascular Imaging Research Center
- Viswanath Ronen Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services
Bone Quality Research Lab
185 Berry Street, Suite 350
San Francisco CA 94107
Phone: (415) 353-4534
Fax: (415) 353-9423
Email: [email protected]

