Translational Metabolic Imaging Lab | Sriram, Kurhanewicz, Peehl
Our Faculty
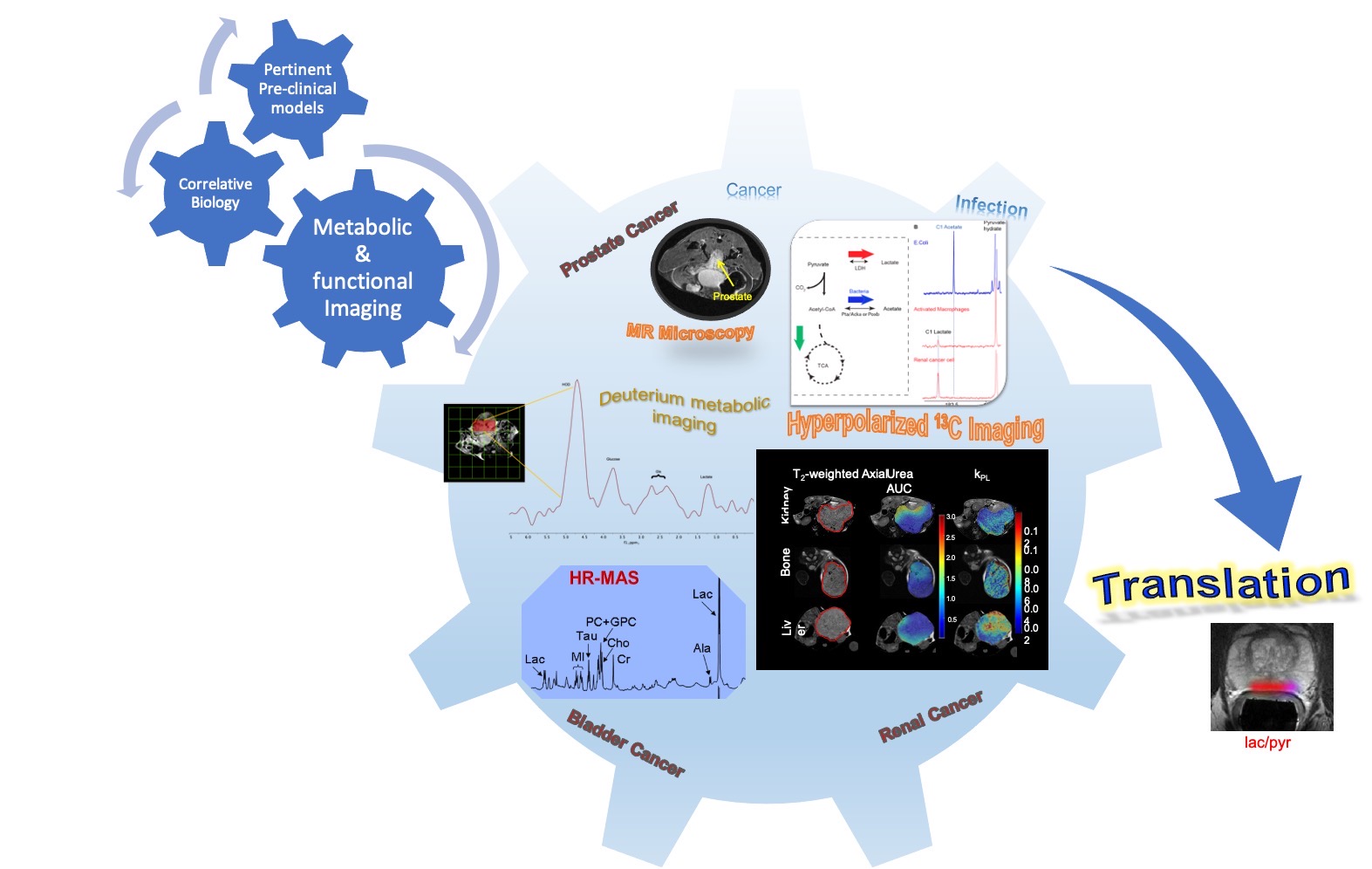
Dr. Renuka Sriram's research work is centered on exploiting the metabolic dysregulation in cancer to develop biomarkers for diagnoses and therapeutic efficacy using magnetic resonance technology. This encompasses the spectrum of magnetic resonance tools from stable isotope resolved metabolomics for identification of dysregulated metabolic pathways, optimization of appropriate bioprobes and its validation using hyperpolarized carbon-13 imaging. With a keen focus on developing robust translatable biomarkers, her lab is focused on establishing a clinically pertinent compendium of models amenable for metabolic imaging and associated engineering controls from ex vivo cell and tissue slice cultures adapted for physiologically relevant MR/PET compatible bioreactor systems all the way to in vivo transgenic and orthotopic murine models using patient derived living tissues. Recent foray into the infectious disease space, has further capitalized on the lab's expertise to identify bacteria specific metabolic pathways as a mechanism to identify infectious pathogens using the cutting edge hyperpolarized 13C MRI.
Dr. Donna M. Peehl received her B.S. degree from Stanford University in Biology and her Ph.D. in Molecular, Cellular and Developmental Biology at the University of Colorado, Boulder, under the mentorship of Dr. Richard Ham, a pioneer in the development of defined media for the culture of mammalian cells. Under the direction of Dr. Eric Stanbridge and Dr. J. Michael Bishop during her postdoctoral fellowships at UC Irvine and UCSF, respectively, Dr. Peehl studied the roles of tumor suppressor genes and oncogenes in human malignancies. During her 35 years as the principal investigator of a basic science laboratory in the Department of Urology at Stanford University, Dr. Peehl’s research focused on the development and application of realistic and representative preclinical models of prostate and kidney cancers, including primary cultures of epithelial and stromal cells, epithelial and stromal co-cultures, immortalized cell lines, induced pluripotent cells, spheroid cultures, and spheroid-derived xenografts. Novel ex vivo – in vivo experimental models based on thin, precision-cut slices of malignant and normal tissue are proving especially useful for developing new therapeutic and imaging approaches for cancer. Tissues are maintained intact as “tissue slice cultures” (TSCs) ex vivo or are implanted under the renal capsule of immunodeficient mice to establish patient-derived xenografts (PDXs) in vivo. Both models faithfully maintain normal and cancer cell and tissue structure and function over time and enable unique experimental opportunities that are provided by few other models. As an Adjunct Professor at UCSF, Dr. Peehl works with her colleagues to use these and other patient-derived preclinical models to test therapeutic agents and optimize hyperpolarized metabolic imaging for genitourinary cancers.
Dr. John Kurhanewicz, is a Professor in Residence in the Departments of Radiology and Biomedical Imaging, Urology and Pharmaceutical Chemistry at University of California, San Francisco, and a member of the California Institute for Quantitative Biology and UCSF Cancer Center, and faculty in the UCSF-UCB Bioengineering Graduate Group. He is the Director of the UCSF Body Research Interest Group, the Biomedical NMR lab, and the Kurhanewicz Laboratory at UCSF. He is currently involved in the development and clinical translation of an extraordinary new molecular imaging technique utilizing hyperpolarized 13C labeled metabolic substrates that has the potential to revolutionize the way MR imaging is used in the risk assessment of prostate cancer patients. He led the first clinical trial of this technology at UCSF and is involved in three ongoing clinical trials investigating its clinical utility.
Current Members
 Shubhangi Agarwal, PhD is a postdoctoral scholar at the Department of Radiology and Biomedical Imaging at UCSF. Dr. Agarwal received her Ph.D. in Biomedical Engineering from the Arizona State University in 2017, where she demonstrated the importance of measuring tumor oxygenation to determine the potential success of hypoxia targeted therapies, using novel oxygen imaging MR techniques. Her research interests lie in using MR imaging techniques to determine predictive cancer biomarkers in patient subpopulations as well as studying the treatment response. Her current work at UCSF is focused on developing metastatic pre-clinical models of small-cell neuroendocrine prostate cancer and evaluating the alterations in metabolism seen in response to therapy, using Hyperpolarized 13C MR imaging techniques. Her other research projects include: 1) Distinguishing metabolic signals of liver tumors from surrounding liver cells using hyperpolarized 13C MRI and Gadoxetate, 2) MR imaging of tuberous sclerosis complex in kidneys.
Shubhangi Agarwal, PhD is a postdoctoral scholar at the Department of Radiology and Biomedical Imaging at UCSF. Dr. Agarwal received her Ph.D. in Biomedical Engineering from the Arizona State University in 2017, where she demonstrated the importance of measuring tumor oxygenation to determine the potential success of hypoxia targeted therapies, using novel oxygen imaging MR techniques. Her research interests lie in using MR imaging techniques to determine predictive cancer biomarkers in patient subpopulations as well as studying the treatment response. Her current work at UCSF is focused on developing metastatic pre-clinical models of small-cell neuroendocrine prostate cancer and evaluating the alterations in metabolism seen in response to therapy, using Hyperpolarized 13C MR imaging techniques. Her other research projects include: 1) Distinguishing metabolic signals of liver tumors from surrounding liver cells using hyperpolarized 13C MRI and Gadoxetate, 2) MR imaging of tuberous sclerosis complex in kidneys.

Will Byrne, Staff Scientist Assistant
 Hecong Qin is a Ph.D. candidate at the UC Berkeley-UCSF joint bioengineering graduate program. His current research focuses on the clinical translation of hyperpolarized 13C MR for simultaneous metabolic and perfusion imaging, with the goal to improve cancer diagnosis and therapeutic monitoring for patients. His other research projects include 1) interrogate in vivo redox capacity using ascorbate-derived hyperpolarized MR and positron emission tomography (PET) imaging probes, 2) investigate lactate metabolism and transport to assess cancer biological behavior and aggressiveness using diffusion-weighted hyperpolarized MR. During graduate school, Hecong was awarded with Genetech Foundation Fellowship and Fletcher Jones Fellowship.
Hecong Qin is a Ph.D. candidate at the UC Berkeley-UCSF joint bioengineering graduate program. His current research focuses on the clinical translation of hyperpolarized 13C MR for simultaneous metabolic and perfusion imaging, with the goal to improve cancer diagnosis and therapeutic monitoring for patients. His other research projects include 1) interrogate in vivo redox capacity using ascorbate-derived hyperpolarized MR and positron emission tomography (PET) imaging probes, 2) investigate lactate metabolism and transport to assess cancer biological behavior and aggressiveness using diffusion-weighted hyperpolarized MR. During graduate school, Hecong was awarded with Genetech Foundation Fellowship and Fletcher Jones Fellowship.

Ivina Mali, PhD, Postdoctoral Scholar
 Deepti Upadhyay, Post Doctoral Scholar
Deepti Upadhyay, Post Doctoral Scholar
I am a postdoctoral scholar at the Department of Radiology and Biomedical Imaging at UCSF. I received my Ph.D. from AIIMS, New Delhi, India in 2018. My Ph.D. work focused on metabolic profiling of tissue extracts and bio-fluids to understand the metabolic dysregulation during disease state using NMR spectroscopy. My current research at UCSF focuses on metabolic characterization of renal cell carcinoma pre-clinical models using NMR based metabolomics.
Our Alumnis
Joao Piraquive Agudelo
Emilie Decavel-Bueff
Kayvan Keshari
Bertram Koelsch
Vickie Zhang
Avantika Sinha
Jinny Sun
Jessie Lee
Rahwa Imam
Justin De Los Santos
Jeff Hsiao
Fayyaz Ahamed
Opportunities
- Engineer position for Core
- Staff Research Associate
- Postdoc
- Well defined small projects for Masters thesis
- Rotation opportunities are available to get a feel for the multiple facets of a translational metabolic imaging lab
Publications
Modeling hyperpolarized lactate signal dynamics in cells, patient‐derived tissue slice cultures and murine models.
Ahamed, F.; Van Criekinge, M.; Wang, Z. J.; Kurhanewicz, J.; Larson, P.; Sriram, R.
NMR Biomed. 2021, 13, e4467. PMID: 33415771 https://pubmed.ncbi.nlm.nih.gov/33415771/
Simultaneous Metabolic and Perfusion Imaging Using Hyperpolarized 13C MRI Can Evaluate Early and Dose-Dependent Response to Radiation Therapy in a Prostate Cancer Mouse Model
Qin, H.; Zhang, V.; Bok, R. A.; Santos, R. D.; Cunha, J. A.; Hsu, I.-C.; Santos BS, J. D.; Lee, J. E.; Sukumar, S.; Larson, P. E. Z.; Vigneron, D. B.; Wilson, D. M.; Sriram, R.; Kurhanewicz, J. International Journal of Radiation Oncology*Biology*Physics 2020, 107, 887–896.
PMCID: PMC7381368 https://pubmed.ncbi.nlm.nih.gov/32339646/
Elevated Tumor Lactate and Efflux in High-grade Prostate Cancer demonstrated by Hyperpolarized 13C Magnetic Resonance Spectroscopy of Prostate Tissue Slice Cultures.
Sriram, R.; Van Criekinge, M.; DeLos Santos, J.; Ahamed, F.; Qin, H.; Nolley, R.; Santos, R. D.; Tabatabai, Z. L.; Bok, R. A.; Keshari, K. R.; Vigneron, D. B.; Peehl, D. M.; Kurhanewicz, J. Cancers 2020, 12. PMCID: PMC7139946 https://pubmed.ncbi.nlm.nih.gov/32110965/
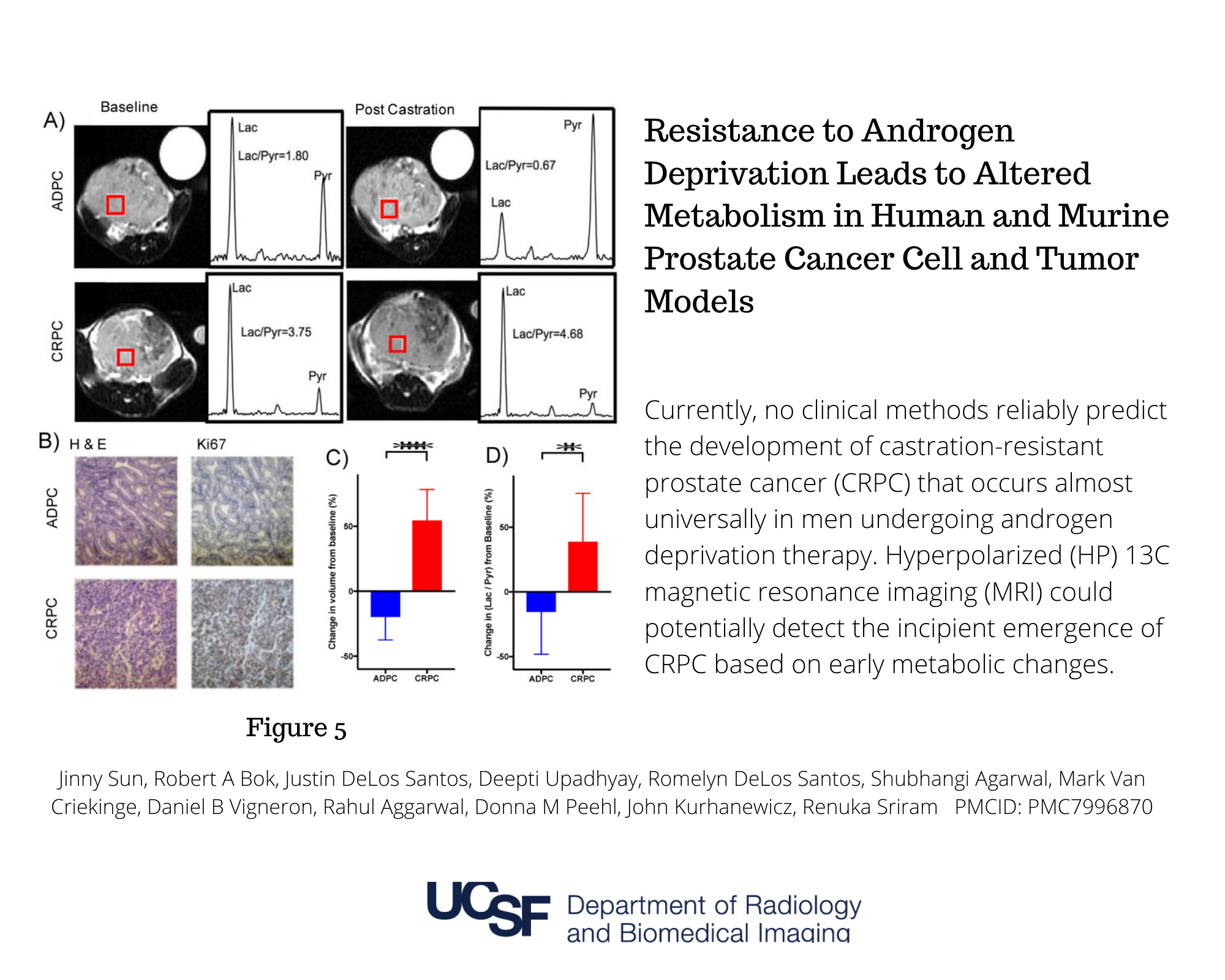
Resistance to Androgen Deprivation Leads to Altered Metabolism in Human and Murine Prostate Cancer Cell and Tumor Models.
Jinny Sun, Robert A Bok, Justin DeLos Santos, Deepti Upadhyay, Romelyn DeLos Santos, Shubhangi Agarwal, Mark Van Criekinge, Daniel B Vigneron, Rahul Aggarwal, Donna M Peehl, John Kurhanewicz, Renuka Sriram PMCID: PMC7996870 https://pubmed.ncbi.nlm.nih.gov/33652703/
Co-Clinical Imaging Research Program (CIRP)
The goal of the Co-Clinical Imaging Research Program (CIRP), (Project Number: 1U24CA253377) is to overcome the translational barrier, to develop co-clinical imaging research resources that will encourage a consensus on how quantitative imaging methods are optimized to improve the quality of imaging results for co-clinical trials. This will be accomplished by using novel quantitative metabolic preclinical hyperpolarized (HP) 13C magnetic resonance imaging (MRI) to assess therapeutic response of small cell neuroendocrine (SCNC) prostate cancer (PCa). Although this proposal will focus on current standard of care treatment, the new quantitative HP 13C metabolic MRI approaches developed in this proposal will have general applicability for a variety of new targeted therapeutic approaches being developed for SCNC as well as for the study of other diseases.
For more information on this program, please visit: https://coclinicalimaging.ucsf.edu/
News
Call for Electronic Posters, 2023 CIRP Annual Virtual Meeting
The NCI Co-Clinical Imaging Research Resources Program (CIRP)- Date: May 03-04, 2023
- Venue: WebEx Meeting
- Register for this Event
- Download flyer
Apply for CIRP Associate Membership
- Cancer Imaging Program
- To apply, please email the program director of CIRP, Huiming Zhang, PhD
- Download flyer
FEATURED PUBLICATION
Resistance to Androgen Deprivation Leads to Altered Metabolism in Human and Murine Prostate Cancer Cell and Tumor Models. Authors: Jinny Sun, Robert A Bok, Justin DeLos Santos, Deepti Upadhyay, Romelyn DeLos Santos, Shubhangi Agarwal, Mark Van Criekinge, Daniel B Vigneron, Rahul Aggarwal, Donna M Peehl, John Kurhanewicz, Renuka Sriram PMCID: PMC7996870 https://pubmed.ncbi.nlm.nih.gov/33652703/
Translational Metabolic Imaging Lab Faculty
- Research Directory
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Quantitative Biomarkers & AI in MSK Imaging
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- Vascular Imaging Research Center
- Viswanath Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services
Contact
- Dr. Renuka Sriram
[email protected] - Dr. Donna Peehl
[email protected] - Dr. John Kurhanewicz
[email protected]