Michael Evans' Lab
Translational research at the interface of chemical biology and nuclear medicine
The scientific premise of the Evans lab is that there are natural, and largely untapped synergies between chemical biology and nuclear medicine. For instance, molecular profiling technologies developed, refined, and applied by chemical biologists (e.g. proteomics, metabolomics) routinely glean biological discoveries that can be exploited by the nuclear medicine community to improve the detection and treatment of major public health challenges. Conversely, researchers in nuclear medicine have the expertise to render new biological discoveries into translational tools for quantititively studying biology in humans with nuclear imaging (e.g. PET, SPECT) and treating disease with therapeutic radioisotopes. In the Evans lab, we aim to breach this gap between chemical biology and nuclear medicine.
Research in the Evans laboratory is focused on new biomarker discovery and development for nuclear imaging and therapeutic applications, primarily in oncology. We are a lab of chemical biologists that interact closely with a diverse set of collaborators, including radiochemists, biomedical engineers, radiologists, and medical oncologists, to develop and translate into humans new imaging tools and therapies. Training opportunities in the lab are by nature interdisciplinary, and we recruit talented young scientists from a wide variety of disciplines to meet the special challenges embedded within the field of biomarker development. Our lab is located on the 5th floor of Genentech Hall on the Mission Bay campus of UCSF, a vibrant academic community in close proximity to several close collaborators (e.g. Professors Jim Wells, Charly Craik, Adam Renslo). Please continue to read below for several examples of projects at the interface of chemical biology and nuclear medicine.
People
 Michael J. Evans, PhD, is a Professor in Residence in the UCSF Department of Radiology and Biomedical Imaging. He is a chemical biologist with an interest in biomarker discovery with proteomics, nuclear medicine, theranostics, and molecular imaging. Dr. Evans earned a BA in Chemistry from St. Mary’s College of Maryland and he obtained his PhD in Organic Chemistry from The Scripps Research Institute (CA) under the supervision of Professor Benjamin Cravatt. This was followed by a postdoctoral fellowship in Molecular Imaging from the Memorial Sloan Kettering Cancer Center in New York under the supervision of Professors Charles Sawyers and Jason Lewis. In 2013, Dr. Evans accepted a faculty position at UCSF.
Michael J. Evans, PhD, is a Professor in Residence in the UCSF Department of Radiology and Biomedical Imaging. He is a chemical biologist with an interest in biomarker discovery with proteomics, nuclear medicine, theranostics, and molecular imaging. Dr. Evans earned a BA in Chemistry from St. Mary’s College of Maryland and he obtained his PhD in Organic Chemistry from The Scripps Research Institute (CA) under the supervision of Professor Benjamin Cravatt. This was followed by a postdoctoral fellowship in Molecular Imaging from the Memorial Sloan Kettering Cancer Center in New York under the supervision of Professors Charles Sawyers and Jason Lewis. In 2013, Dr. Evans accepted a faculty position at UCSF.
Dr. Evans has published over 80 peer-reviewed articles, 40 meeting abstracts, and is a co-inventor on 13 patents pending or issued. Dr. Evans also is the principle investigator or co-PI on several human trials focused on new strategies for imaging and treating tumors with radiopharmaceuticals. He is a scientific co-founder and previously served on the scientific advisory board of ORIC Pharmaceuticals, Inc. He is a co-founder of Therapaint, Inc, Honeybear Biosciences, and Hap10, Inc. Dr. Evans is a member of the American Chemical Society, the Society of Nuclear Medicine and Molecular Imaging, and the World Molecular Imaging Society. He has been invited nationally and internationally to lecture about his research. Has been recognized with numerous honors, including a Young Investigator Award from the Prostate Cancer Foundation, a Pathway to Independence Award from the National Cancer Institute, a Research Scholar Award from the American Chemical Society, the 2023 Roger Tsien Award for excellence in chemical biology from the World Molecular Imaging Society, and he was a 2020 inductee to the Council of Distinguished Investigators in the Academy of Radiology and Biomedical Imaging Research.
 Yifan Cui, PhD is a postdoctoral fellow in the Evans lab. He received his B.S. in Chemistry from Soochow University in 2016. Then, he earned his Ph.D. in Organic Chemistry from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (SIOC, CAS) in 2021 under the supervision of Prof. Shengming Ma. During his Ph.D. study, he developed several transition-metal catalyzed functionalization reactions of amines.
Yifan Cui, PhD is a postdoctoral fellow in the Evans lab. He received his B.S. in Chemistry from Soochow University in 2016. Then, he earned his Ph.D. in Organic Chemistry from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (SIOC, CAS) in 2021 under the supervision of Prof. Shengming Ma. During his Ph.D. study, he developed several transition-metal catalyzed functionalization reactions of amines.
 Luke Jaskowski is a Chemistry and Chemical Biology graduate student in the Evans lab. He received his B.S. in Chemistry from the University of Alabama at Birmingham in 2022, completing his honors thesis under the direction of Dr. Suzanne Lapi. He worked on fluorine-18 radiolabeling of anticancer natural products known as taccalonolides to evaluate their biodistribution and overall therapeutic potential. Upon joining the Evans lab in 2024, he has focused on surveying mechanisms of resistance to radiation therapy, hoping to develop treatment strategies which overcome resistance.
Luke Jaskowski is a Chemistry and Chemical Biology graduate student in the Evans lab. He received his B.S. in Chemistry from the University of Alabama at Birmingham in 2022, completing his honors thesis under the direction of Dr. Suzanne Lapi. He worked on fluorine-18 radiolabeling of anticancer natural products known as taccalonolides to evaluate their biodistribution and overall therapeutic potential. Upon joining the Evans lab in 2024, he has focused on surveying mechanisms of resistance to radiation therapy, hoping to develop treatment strategies which overcome resistance.
 Kayden Kwah, PhD is a Postdoctoral Fellow in the Evans Lab at UCSF Radiology & Biomedical Imaging, where he focuses on developing and translating novel imaging and radiopharmaceutical technologies. He received his BSc (Honours I) from the Queensland Institute of Medical Research – The University of Queensland, and then completed his PhD at Mater Research – The University of Queensland under the supervision of Prof. John Hooper. During his doctoral studies, Kayden developed and characterised an innovative antibody-drug conjugate cocktail therapy for solid cancers. His training spans recombinant protein production, biophysical and molecular analyses, cryogenic electron microscopy, and both in vitro and in vivo drug efficacy characterisation.
Kayden Kwah, PhD is a Postdoctoral Fellow in the Evans Lab at UCSF Radiology & Biomedical Imaging, where he focuses on developing and translating novel imaging and radiopharmaceutical technologies. He received his BSc (Honours I) from the Queensland Institute of Medical Research – The University of Queensland, and then completed his PhD at Mater Research – The University of Queensland under the supervision of Prof. John Hooper. During his doctoral studies, Kayden developed and characterised an innovative antibody-drug conjugate cocktail therapy for solid cancers. His training spans recombinant protein production, biophysical and molecular analyses, cryogenic electron microscopy, and both in vitro and in vivo drug efficacy characterisation.
At the Evan's lab, Kayden aims to integrate his expertise in protein engineering, biophysical/structural analysis, and translational biology to advance new strategies for theranostics in solid cancers. He works closely with chemists to drive the development of precision diagnostics and targeted therapeutics. Outside the lab, Kayden enjoys exploring specialty coffee and is a self-proclaimed audiophile.
 Jesse Ling, PhD is a postdoctoral fellow in the Evans lab. He received his BSc and PhD in organic chemistry from the University of Hong Kong, under the supervision of Prof. Pauline Chiu. He pursued further training at Tufts University in Prof. Clay S. Bennett's group, focusing on glycosciences. After a brief period working in Hong Kong during the Pandemic, he joined the Evans lab in 2022. He has diversified research interests in synthetic chemistry and chemical biology.
Jesse Ling, PhD is a postdoctoral fellow in the Evans lab. He received his BSc and PhD in organic chemistry from the University of Hong Kong, under the supervision of Prof. Pauline Chiu. He pursued further training at Tufts University in Prof. Clay S. Bennett's group, focusing on glycosciences. After a brief period working in Hong Kong during the Pandemic, he joined the Evans lab in 2022. He has diversified research interests in synthetic chemistry and chemical biology.
 Apurva Pandey, PhD is a Postdoctoral Fellow in the Evans lab. She obtained her B.Sc.(H) (2014) and M.Sc. (2016) at the University of Delhi, India. In 2021, she obtained her Ph.D. in Chemistry from Stony Brook University, New York, under the supervision of Dr. Eszter Boros. During her Ph.D. she worked on developing, synthesizing, and testing metallo-siderophore-antibiotic conjugates as theranostic probes for bacterial infections. Apurva is a trained radiochemist and has first/co-authored 8 peer-reviewed articles and a book chapter. In the Evans lab, she is currently working on the Granzyme project, developing, and testing Granzyme specific probes as potential diagnostic tools for bacterial and viral infections. Apurva is a proud Ravenclaw, when not in lab she can be found watching space videos, cooking, and collecting Harry Potter merchandise.
Apurva Pandey, PhD is a Postdoctoral Fellow in the Evans lab. She obtained her B.Sc.(H) (2014) and M.Sc. (2016) at the University of Delhi, India. In 2021, she obtained her Ph.D. in Chemistry from Stony Brook University, New York, under the supervision of Dr. Eszter Boros. During her Ph.D. she worked on developing, synthesizing, and testing metallo-siderophore-antibiotic conjugates as theranostic probes for bacterial infections. Apurva is a trained radiochemist and has first/co-authored 8 peer-reviewed articles and a book chapter. In the Evans lab, she is currently working on the Granzyme project, developing, and testing Granzyme specific probes as potential diagnostic tools for bacterial and viral infections. Apurva is a proud Ravenclaw, when not in lab she can be found watching space videos, cooking, and collecting Harry Potter merchandise.
 Junyang Qi, PhD is currently a postdoctoral fellow in the Evans lab. She obtained her PhD in medicinal chemistry from Sun Yat-sen University in 2021 under the supervision of Prof. Wenbin Deng. Following that, she continued to pursue a two-year postdoctoral training with Prof. Wenbin Deng and Prof. Lin Mei. Her research interests lie in medicinal chemistry and nanomedicine. Throughout her PhD and postdoctoral trainings, she focused on developing functionalized black phosphorus nanomaterials and small-molecule probes for cancer theranostics. She joined the Evans lab in 2024 to expand her research to the field of radiotherapy and work on developing radio-based ligands and probes for achieving precise cancer theranostics.
Junyang Qi, PhD is currently a postdoctoral fellow in the Evans lab. She obtained her PhD in medicinal chemistry from Sun Yat-sen University in 2021 under the supervision of Prof. Wenbin Deng. Following that, she continued to pursue a two-year postdoctoral training with Prof. Wenbin Deng and Prof. Lin Mei. Her research interests lie in medicinal chemistry and nanomedicine. Throughout her PhD and postdoctoral trainings, she focused on developing functionalized black phosphorus nanomaterials and small-molecule probes for cancer theranostics. She joined the Evans lab in 2024 to expand her research to the field of radiotherapy and work on developing radio-based ligands and probes for achieving precise cancer theranostics.
 Haibo Xu, PhD is a postdoctoral fellow in the Evans lab. He received his B.S. in Chemistry from Zhejiang University of Technology in 2017. Then, he earned his Ph.D. in Organic Chemistry from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences in 2023 under the supervision of Prof. Shengming Ma. During his Ph.D. he focus on the synthesis of chiral amines and Eight-membered cycles. After short-term working as research assistant in Fudan University, he joined the Evans lab in 2024. His research interests involve the chemical synthesis and molecular imaging.
Haibo Xu, PhD is a postdoctoral fellow in the Evans lab. He received his B.S. in Chemistry from Zhejiang University of Technology in 2017. Then, he earned his Ph.D. in Organic Chemistry from Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences in 2023 under the supervision of Prof. Shengming Ma. During his Ph.D. he focus on the synthesis of chiral amines and Eight-membered cycles. After short-term working as research assistant in Fudan University, he joined the Evans lab in 2024. His research interests involve the chemical synthesis and molecular imaging.
News
January 2025: Congrats to co-lead author Apurva Pandey and our collaborators in the Craik and Verba labs at UCSF for landing the cover art in this month’s issue of Cancer Research.
January issue of Clinical Cancer Research, Volume 85, Issue 2
https://aacrjournals.org/cancerres/issue/85/2

May 2024: Dr. Michael Evans has been awarded the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Inaugural Sam Gambhir, MD Trailblazer Award, which honors outstanding achievement and excellence in transformative research and exceptional mentorship.
April 2024: Dr. Apurva Pandey received a speaker award for her presentation at the UCSF Precision Imaging of Cancer and Therapy (PICT) retreat.
January 2024: The Evans lab, in collaboration with Drs. Charles Craik and Rahul Aggarwal, receive an R01 from NCI entitled “Maximizing tumor responses to targeted radiotherapy with a conditionally activated membrane binding probe”.
October 2023: Dr. Apurva Pandey is awarded a highly prestigious Jeff and Loyd Zisk Young Investigator Award from the Prostate Cancer Foundation. She is one of 25 awardees in the 2023 class and was recognized for her project entitled “Conditionally Activated Membrane Binding Probes for Improved Targeted Radiotherapy in Metastatic Castration Resistant Prostate Cancer.” She will be jointly mentored by Drs. Evans, Charles Craik, and Felix Feng of UCSF.

September 2023: Dr. Michael Evans receives the 2023 Roger Tsien Award for Excellence in Chemical Biology from the World Molecular Imaging Society. A recording of the plenary lecture can be found through this link:
https://www.youtube.com/watch?v=tQ7fhteBMS4&t=700s
August 2023: Drs. Shalini Chopra, Apurva Pandey and Garima Arvikar were selected as “Ones to Watch” by the Society of Nuclear Medicine and Molecular Imaging. This distinction recognizes rising stars in the field of nuclear medicine research.

July 2023: Dr. Michael Evans was promoted to Professor in the Department of Radiology and Biomedical Imaging at UCSF.
July 2023: The Evans lab, in collaboration with Drs. Lawrence Fong and Rahul Aggarwal, receive an R01 from the National Cancer Institute entitled “Combining immunotherapy with molecularly targeted radiation therapy”.
June 2023: Dr. Shalini Chopra was awarded a Young Investigator Prize at the Society of Nuclear Medicine and Molecular Imaging in Chicago IL. She was recognized for her abstract entitled: “Imaging granzyme biochemistry in CAR T cell immunotherapy with restricted interaction peptide and PET”
June 2023: Dr. Shalini Chopra co-authors a manuscript in ACS Central Science with Drs. Lei Wang and Paul Klauser entitled “Covalent Proteins as Targeted Radionuclide Therapies Enhance Antitumor Effects”.
May 2023: A first in human clinical trial opens at UCSF to evaluate 64Cu-GRIP B as a marker of antitumor immunity in cancer patients. Details can be found by following the link:
https://clinicaltrials.gov/study/NCT05888532?term=granzyme%20B&rank=1
April 2023: Dr. Shalini Chopra publishes a first author manuscript entitled “Theranostic Targeting of CUB Domain-Containing Protein 1 (CDCP1) in Multiple Subtypes of Bladder Cancer.” The article was featured on the cover of the April issue of Clinical Cancer Research and was highlighted by several news outlets.
April issue of Clinical Cancer Research
Press release from the Society of Nuclear Medicine and Molecular Imaging, June 26, 2023:
https://www.snmmi.org/NewsPublications/NewsDetail.aspx?ItemNumber=44219

Press release from Urotoday, January 19, 2023:
October 2022: Dr. Apurva Pandey is awarded a poster prize at the 2022 World Molecular Imaging Congress in Miami FL. She received the award in recognition of her research investigating granzyme mediated immune responses to viral and bacterial infections.
July 2022: The Evans lab, in collaboration with Dr. Rahul Aggarwal of UCSF, is awarded a Translational Science Award from the Congressionally Directed Medical Research Programs. The project is entitled “Granzyme B PET as a Novel Means to Assess T cell Activation in Response to 177Lu-PSMA-617 Priming in Metastatic Castration Resistant Prostate Cancer”.
May 2022: Dr. Yangjie Huang received the Alavi Mandell award for the paper titled “The Synthesis and Structural Requirements for Measuring Glucocorticoid Receptor Expression In Vivo with (6)-11CYJH08 PET” which appeared in the May issue of the Journal of Nuclear Medicine. The Alavi-Mandell Award recognizes outstanding contributions to the field from young scientists, fellows, or physician residents. Congratulations to Dr. Huang on this prestigious award!
January 2022: The Evans lab receives a Mission Boost Award from the American Cancer Society to translate 18F-TRX into cancer patients to measure iron dysregulation in tumors.
July 2021: Dr. Ning Zhao was recognized by the Society of Nuclear Medicine and Molecular Imaging’s “Ones to watch” campaign. SNMMI’s Ones to Watch campaign aims to recognize those with the potential to shape the future of precision medicine across all spectrums of the field. Congratulations to Dr. Zhao on this prestigious honor!
July 2021: Our new article titled “Ferronostics: Measuring Tumoral Ferrous Iron with PET to Predict Sensitivity to Iron-Targeted Cancer Therapies” appeared in the July issue of the Journal of Nuclear Medicine. Congratulations to first author Dr. Ning Zhao!
July 2021: The Evans lab, in collaboration with Dr. Adam Renslo, was awarded a pilot grant from the Benioff Institute for Prostate Cancer Research. The project is titled “Exploiting ferroaddiction in castration resistant prostate cancer for precision therapy”.
July 2021: The Evans lab, in collaboration with Dr. Adam Renslo, was awarded a four year R01 from the National Institute of Biomedical Imaging and Bioengineering. The project is titled “Developing a pre-targeting strategy to detect Fe(II) for nuclear medicine applications”.
June 2021: The Evans lab receives a REAC award from UCSF to support the purchase of new HPLC equipment for radiochemistry development at the Center for Functional and Molecular Imaging.
June 2021: The Evans lab, in collaboration with Drs. James Wells and Rahul Aggarwal, was awarded a three year Idea Development Award from the CDMRP Prostate Cancer Research Program. The project, titled “Exploring CUB Domain-Containing Protein 1 as a Theranostic Target for Castration-Resistant Prostate Cancer” aims to develop a new radioligand for prostate cancer treatment.
May 2021: Our clinical trial with Dr. Rahul Aggarwal evaluating 11C-YJH08 PET in prostate cancer patients is now open and accepting patients.
April 2021: The Evans lab, in collaboration with Drs. Charles Craik and David Wilson, was awarded a four year R01 from the National Institute of Allergy and Infectious Diseases. The project is titled “New radiotracer development to target immune cell mobilization of granzyme proteolytic activity”.
February 2021: The Evans lab, in collaboration with Drs. Charles Craik, Rahul Aggarwal, and Lawrence Fong, was awarded a five year R01 from the National Cancer Institute. The project is titled “Precision targeting of T cell cytotoxicity with PET”
December 2020: Our new article entitled “An analysis of isoclonal antibody formats suggests a role for measuring PD-L1 with low molecular weight PET radiotracers” appeared in the December issue of Molecular Imaging and Biology. Congratulations to co-first authors Dr. Junnian Wei and Yung-hua Wang!
November 2020: Congratulations to Dr. Shalini Chopra for receiving an award for her presentation at the 2020 Society of Nuclear Medicine India annual international meeting!
August 2020: Congratulations to Drs. Yangjie Huang and Junnian Wei for accepting academic appointments in the chemistry departments of Minjiang University and Peking University, respectively.
July 2020: Dr. Evans was awarded a Dean’s Apple award from the UCSF School of Pharmacy for excellence in teaching.
June 2020: Dr. Evans presents an abstract titled “CDCP1 is a novel target for radioligand therapy in metastatic castration resistant prostate cancer refractory to treatment with PSMA directed radioligands”. The abstract was also presented by Dr. Heiko Schroder of MSKCC in the Oncology highlights session!
June 2020: Dr. Evans receives the Distinguished Investigator Award from the Academy for Radiology and Biomedical Imaging Research.
June 2020: The Evans lab was awarded an Idea Expansion Award from the CDMRP Prostate Cancer Research Program to study glucocorticoid receptor expression in prostate cancer patients with 11C-YJH08, a novel radioligand developed in the lab.
May 2020: Our new article “The synthesis and structural requirements for measuring glucocorticoid receptor expression in vivo with (±)-11C-YJH08 PET” appeared in the May issue of the Journal of Nuclear Medicine. Congratulations to first author Dr. Yangjie Huang!
April 2020: "A novel radioligand reveals tissue specific pharmacological modulation of glucocorticoid receptor expression with positron emission tomography" is now online at ACS Chemical Biology. Congratulations to first author Yangjie Huang!
April 2020: "Theranostic targeting of CUB domain containing protein 1 (CDCP1) in pancreatic cancer" is now online at Clinical Cancer Research. Congratulations to first author Anna Moroz!
March 2020: A recent paper published in Molecular and Cellular Proteomics outlining how mTORC1 remodels the cell surfaceome was highlighted in ASBMB Today. Congratulations to first authors Junnian Wei (Evans lab) an Kevin Leung (Wells lab).
January 2020: The Evans lab, in collaboration with David Oh’s lab at UCSF, was awarded a one year grant from the Marcus Program in Precision Medicine Innovation, Seeding Bold Ideas Initiative. The project aims to develop a new biomarker to study immune cell activation in vivo with PET.
December 2019: The Evans lab was awarded a new grant from the LAM Foundation to study iron dysregulation with PET in patients. This is on of the few human PET trials currently accruing in the world for LAM patients, and aims to better detect this multisystem disease with a radiotracer targeting ferric iron uptake through the transferrin receptor. This project is a collaboration with Professor Steven Ruoss, MD of Stanford University.
January 2019: The article "Imaging PD-L1 expression with immunoPET" was recognized by the American Chemical Society as one of the top five most read articles in Bioconjugate Chemistry."

January 2018: "Imaging PD-L1 expression with immunoPET" is the most downloaded article within the past 30 days on the website for Bioconjugate Chemistry, and the article is featured on the cover of the January 2018 edition of BC (see above).
January 2018: An manuscript in eLife describing our recent collaboration with the Wells lab at USCF is now online (https://elifesciences.org/articles/31098). This exciting research applied proteomics to define new actionable cell surface drug targets to treat and specifically detect in vivo with PET cancer cells with hyperactive KRAS. This work has been highlighted by several news outlets:
http://ecancer.org/news/13218-new-approach-attacks--undruggable--cancers-from-the-outside-in.php
December 2017: "Real time transferrin based PET detects MYC positive prostate cancer" was selected by the editors of Molecular Cancer Research as a part of the "Must Read Collection" for 2017. The article will be featured at the 2018 AACR national meeting.
November 2017: The Evans lab was awarded a R01 from the National Institute of Mental Health to develop a new fluorine-18 labeled radioligand to measure glucocorticoid receptor expression in CNS disorders. The three year grant will culminate in a first in human trial at UCSF!
November 2017: The Evans lab was awarded a Research grant from the Tuberous Sclerosis Alliance to apply proteomics to discover new biomarkers to better image and treat clinically problematic tumors arising from the childhood disorder tuberous sclerosis complex.
September 2017: Research from the Evans lab to develop imaging biomarkers for PET to measure MYC signaling non-invasively in human tumors was featured on the Congressionally Directed Medical Research Program website: http://cdmrp.army.mil/pcrp/research_highlights/17evans_highlight
June 2017: Dr. Michael Evans' lab received an American Brain Tumor Association Discovery Award to continue studying 68Ga-citrate PET/MR in patients with high grade glioma. The aim of this project is to improve the detection of high grade glioma by measuring mTORC1 signaling in the tumor with 68Ga, a Fe(III) biomimetic that targets the transferrin receptor on tumor cells.
April 2017: Congratulations to former undergraduate researcher Sophie Shen (UC Berkeley) for being accepted into a summer research fellowship at FibroGen, Inc., a bay area biotech company.
April 2017: Congratulations to former undergraduate researcher and technician Loc Huynh for his admission into the PhD program in the Department of Chemistry at University of Florida, as well as receiving the Grinter Fellowship to supplement his stipend.
April 2017: Dr. Michael Evans' lab received an American Cancer Society Research Scholar Award to continue studying 89Zr-labeled transferrin, a radiotracer for PET that measures mTORC1 signaling in tumor cells non-invasively with PET.
January 2017: Congratulations to Dr. Matthew Parker for receiving a postdoctoral fellowship from the Department of Defense Prostate Cancer Research Program. His grant is titled "A Novel Prodrug Strategy to Treat Prostate Cancer by Targeting MYC-Driven Nucleotide Biosynthesis". Dr. Parker is also co-mentored by Professor Davide Ruggero at UCSF.
January 2017: Research from the Evans lab was recognized as the Featured Translational Article in the current issue of the Journal of Nuclear Medicine. http://jnm.snmjournals.org/content/58/1/81
December 2016: Congratulations to Dr. Charles Truillet on accepting a Professorship in the Department of Biomedical Imaging at the French Alternative Energies and Atomic Energy Commission (CEA).
January 2016: Congratulations to Dr. Charles Truillet for receiving a postdoctoral fellowship from the Department of Defense Prostate Cancer Research Program. His grant is titled "Development of radiolabeled transferrin constructs to detect and treat castration resistant prostate cancer." Dr. Truillet is also co-mentored by Dr. Davide Ruggero of UCSF.
2015 SNMMI Highlioghts Lecture, Part 1
Non-Invasive Biomarkers For Prostate Cancer
New Imaging Agent Could Improve Prostate Cancer Diagnosis and Treatment
Opportunities
Post-doctoral Opportunities
The Evans lab is currently seeking exceptional candidates for a postdoctoral appointment focusing on new probe development for nuclear theranostics and studying tumor immunology.
Interested candidates should contact Dr. Evans directly and provide a copy of their CV and references at [email protected].
Graduate Students
Dr. Evans is a member of the Chemistry and Chemical Biology graduate program (https://ccb.ucsf.edu/) and the Pharmaceutical Sciences and Pharmacogenomics graduate program (https://pspg.ucsf.edu/) at UCSF. The lab is openly accepting students. Students interested in rotations should contact Dr. Evans directly via email.
Staff
Employment Opportunities for staff positions are posted through the UCSF employment websites
Publications

Examples of cover art from Evans lab publications. A complete list of Evans lab publications can be found at Michael Evans' UCSF profiles page.
Alumni
Former postdoctoral fellows:
 Faraaz Aziz is a third year undergraduate student at UC Berkeley majoring in chemistry. He joined the Evans lab in June 2024 and has worked with post-docs on the synthesis of small molecules. After graduation he hopes to go to medical school. Outside of academics, Faraaz enjoys cooking and playing the violin as part of the UC Berkeley Philharmonic Orchestra.
Faraaz Aziz is a third year undergraduate student at UC Berkeley majoring in chemistry. He joined the Evans lab in June 2024 and has worked with post-docs on the synthesis of small molecules. After graduation he hopes to go to medical school. Outside of academics, Faraaz enjoys cooking and playing the violin as part of the UC Berkeley Philharmonic Orchestra.
 Zhuo Chen, PhD earned her B.S. in Materials Chemistry from Tianjin University, China, and a M.S. in Chemistry (specializing in polymer science) from the University of Massachusetts Lowell, MA. In 2018, she received her Ph.D. in Chemistry under the supervision of Professor Jeremiah Gassensmith at the University of Texas at Dallas, where she focused on functionalizing virus-like particles as a platform for stimuli-responsive drug delivery and fluorescence imaging. She is a co-author on 8 manuscripts in well-respected peer reviewed journals like Small, Bioconjugate Chemistry, and Chemical Communications. She has extensive experience in nanoparticle-based therapeutics development and formulation, bioconjugation chemistry and organic synthesis.
Zhuo Chen, PhD earned her B.S. in Materials Chemistry from Tianjin University, China, and a M.S. in Chemistry (specializing in polymer science) from the University of Massachusetts Lowell, MA. In 2018, she received her Ph.D. in Chemistry under the supervision of Professor Jeremiah Gassensmith at the University of Texas at Dallas, where she focused on functionalizing virus-like particles as a platform for stimuli-responsive drug delivery and fluorescence imaging. She is a co-author on 8 manuscripts in well-respected peer reviewed journals like Small, Bioconjugate Chemistry, and Chemical Communications. She has extensive experience in nanoparticle-based therapeutics development and formulation, bioconjugation chemistry and organic synthesis.
 Shalini Chopra, PhD is a postdoctoral fellow in the Evans lab. She received her B.Sc. (Hons. School) in Biophysics and M.Sc. in Nuclear Medicine from Panjab University, India. She received her Ph.D. in Biophysics from Panjab University under the supervision of Dr. Baljinder Singh, Professor at PGIMER, India. Her Ph.D. project involved development of antimicrobial peptide fragments as potential PET probes for imaging bacterial infections in murine models. She received the DAAD (German Academic Exchange Service) fellowship to carry a part of thesis work under the stewardship of Professor Hans-Jürgen Wester at Technical University of Munich, Germany. She is well versed with molecular imaging techniques (pre-clinical and clinical level), radiolabeling and conjugation strategies for the development of new tracers, and has authored publications in Applied Radiation and Isotopes and Cancer Biotherapies and Radiopharmaceuticals.
Shalini Chopra, PhD is a postdoctoral fellow in the Evans lab. She received her B.Sc. (Hons. School) in Biophysics and M.Sc. in Nuclear Medicine from Panjab University, India. She received her Ph.D. in Biophysics from Panjab University under the supervision of Dr. Baljinder Singh, Professor at PGIMER, India. Her Ph.D. project involved development of antimicrobial peptide fragments as potential PET probes for imaging bacterial infections in murine models. She received the DAAD (German Academic Exchange Service) fellowship to carry a part of thesis work under the stewardship of Professor Hans-Jürgen Wester at Technical University of Munich, Germany. She is well versed with molecular imaging techniques (pre-clinical and clinical level), radiolabeling and conjugation strategies for the development of new tracers, and has authored publications in Applied Radiation and Isotopes and Cancer Biotherapies and Radiopharmaceuticals.
 Yangjie Huang, PhD (Thesis advisor: Zhiqiang Weng, PhD, Fuzhuo University)
Yangjie Huang, PhD (Thesis advisor: Zhiqiang Weng, PhD, Fuzhuo University)
Tenure: 2017 – 2020
Publications: 6 total (2 first author)
Current Position: Distinguished Assistant Professor, Department of Chemistry, Minjiang University, CN
 Hyunjung Kim, PhD received a B.S. in Chemistry from Korea Univ., and a M.S. in Organic Chemistry (program: Bioorganic Medicinal Chemistry) from Yonsei Univ. in Korea. In 2019, she received her Ph.D. in Health Sciences and Technology (program: Radiopharmaceutical Chemistry) under the supervision of Professor Yearn Seong Choe at Sungkyunkwan Univ. in Korea. During her PhD, she carried out synthesis of small molecules for development of molecular probes for brain and tumor angiogenesis PET or PET/optical imaging. She also attended several national and international conferences to give oral and poster presentations and received awards. Dr. Kim is the first or co-author on 7 manuscripts in peer-reviewed journals like ACS Chemical Neuroscience and Scientific Reports.
Hyunjung Kim, PhD received a B.S. in Chemistry from Korea Univ., and a M.S. in Organic Chemistry (program: Bioorganic Medicinal Chemistry) from Yonsei Univ. in Korea. In 2019, she received her Ph.D. in Health Sciences and Technology (program: Radiopharmaceutical Chemistry) under the supervision of Professor Yearn Seong Choe at Sungkyunkwan Univ. in Korea. During her PhD, she carried out synthesis of small molecules for development of molecular probes for brain and tumor angiogenesis PET or PET/optical imaging. She also attended several national and international conferences to give oral and poster presentations and received awards. Dr. Kim is the first or co-author on 7 manuscripts in peer-reviewed journals like ACS Chemical Neuroscience and Scientific Reports.
 Matthew Parker, PhD (Thesis advisor: Christian Schafmeister, PhD, University of Pittsburgh)
Matthew Parker, PhD (Thesis advisor: Christian Schafmeister, PhD, University of Pittsburgh)
Postdoctoral fellow of the CDMRP Prostate Cancer Research Program
Tenure: 2015 – 2017
Publications: 5 total (1 co-first author)
Current Position: Assistant Researcher, David Wilson lab, Department of Radiology and Biomedical Imaging, UCSF
 Fiona Quimby is an honors graduate from Saint Mary's College of California. Recently, earning her BS in Biology. During her undergraduate degree she worked extensively in Dr. Michael Marchetti's lab in which she utilized carbon and nitrogen stable isotope technology in order to study endangered species and help determine allocation of funding. Currently, she works as a Junior Research Associate in the Evans lab while also gaining clinical experience as a medical assistant at a local private practice helping run their onsite lab and processing samples. In the Evans lab she hopes to utilize past knowledge and to learn more about the theranostic approach using radioisotopes and the impact it has on current drug and imaging development.
Fiona Quimby is an honors graduate from Saint Mary's College of California. Recently, earning her BS in Biology. During her undergraduate degree she worked extensively in Dr. Michael Marchetti's lab in which she utilized carbon and nitrogen stable isotope technology in order to study endangered species and help determine allocation of funding. Currently, she works as a Junior Research Associate in the Evans lab while also gaining clinical experience as a medical assistant at a local private practice helping run their onsite lab and processing samples. In the Evans lab she hopes to utilize past knowledge and to learn more about the theranostic approach using radioisotopes and the impact it has on current drug and imaging development.
 Peng Wen Tan, PhD received his B.Sc. (Hons) in Chemistry and Biological Chemistry from Nanyang Technological University, Singapore. He was awarded A*STAR Graduate (Overseas) Scholarship to pursue his DPhil in Organic Chemistry from University of Oxford, UK under the supervision of Prof. Dr. Darren J. Dixon, UK and Dr. Jayasree Seayad, Singapore. His PhD project involved the development of contemporary transition metal catalyzed reactions and their applications in the total synthesis of natural products. After graduation, he went on to fulfil his service commitment at Institute of Bioengineering and BioImaging, Singapore where he was trained in radiochemistry to develop molecular imaging probes.
Peng Wen Tan, PhD received his B.Sc. (Hons) in Chemistry and Biological Chemistry from Nanyang Technological University, Singapore. He was awarded A*STAR Graduate (Overseas) Scholarship to pursue his DPhil in Organic Chemistry from University of Oxford, UK under the supervision of Prof. Dr. Darren J. Dixon, UK and Dr. Jayasree Seayad, Singapore. His PhD project involved the development of contemporary transition metal catalyzed reactions and their applications in the total synthesis of natural products. After graduation, he went on to fulfil his service commitment at Institute of Bioengineering and BioImaging, Singapore where he was trained in radiochemistry to develop molecular imaging probes.
 Charles Truillet, PhD (Thesis advisor: Olivier Tillement, PhD, Universite Claude Bernard Lyon 1)
Charles Truillet, PhD (Thesis advisor: Olivier Tillement, PhD, Universite Claude Bernard Lyon 1)
Postdoctoral fellow of the CDMRP Prostate Cancer Research Program
Tenure: 2014 – 2017
Publications: 23 total (5 first author)
Current Position: Assistant Professor, Department of Biomedical Imaging, Atomic Energy Commission, FR
 Junnian Wei, PhD (Thesis advisor: Zhenfeng Xi, PhD, Peking University)
Junnian Wei, PhD (Thesis advisor: Zhenfeng Xi, PhD, Peking University)
Tenure: 2017 – 2020
Publications: 12 total (2 first author)
Current position: Assistant Professor, Department of Chemistry, Peking University, CN
 Alice Whitehurst is a graduate from the Master of Science Biomedical Imaging (MSBI) program in the Department of Radiology and Biomedical Imaging at UCSF. She secured her B.Sc. in biological sciences with an emphasis on microbiology and immunology (2022) from UC Merced. During her undergraduate degree, she studied and researched the ways keratin-8 execute distinct morphogenetic programs to assemble certain embryonic tissues. She hopes to utilize her past knowledge, further expand her understanding of radiochemistry, and acquire skills for in vivo and in vitro studies in the Evans lab. Alice finds comfort in watching latte art videos and attempting to make one herself.
Alice Whitehurst is a graduate from the Master of Science Biomedical Imaging (MSBI) program in the Department of Radiology and Biomedical Imaging at UCSF. She secured her B.Sc. in biological sciences with an emphasis on microbiology and immunology (2022) from UC Merced. During her undergraduate degree, she studied and researched the ways keratin-8 execute distinct morphogenetic programs to assemble certain embryonic tissues. She hopes to utilize her past knowledge, further expand her understanding of radiochemistry, and acquire skills for in vivo and in vitro studies in the Evans lab. Alice finds comfort in watching latte art videos and attempting to make one herself.
 Ning Zhao, PhD (Thesis advisors: Kevin Smith, PhD and Graca Vicente, PhD, Louisiana State University)
Ning Zhao, PhD (Thesis advisors: Kevin Smith, PhD and Graca Vicente, PhD, Louisiana State University)
Postdoctoral fellow of the CDMRP Prostate Cancer Research Program
Named to the 2021 “Ones to watch” list by the Society for Nuclear Medicine and Molecular Imaging
Tenure: 2018 – 2021
Publications: 8 total (4 first author)
Former graduate and undergraduate students:
 Sasank Sakhamuri (MSBI program, Department of Radiology and Biomedical Imaging, UCSF)
Sasank Sakhamuri (MSBI program, Department of Radiology and Biomedical Imaging, UCSF)
 Loc Huynh (College of Chemistry, UC Berkeley)
Loc Huynh (College of Chemistry, UC Berkeley)
Tenure: 2015 – 2017
Publications: 5 total
Current position, PhD candidate (Chemistry), Michael Harris lab, University of Florida, FL
 Khaled Jami (College of Chemistry, UC Berkeley)
Khaled Jami (College of Chemistry, UC Berkeley)
Tenure: 2016 – 2017
Publications: 2 total
Current position: PhD candidate (Chemistry), Dylan Murray lab, University of California Davis, CA
 Nhan Dang (College of Chemistry, UC Berkeley)
Nhan Dang (College of Chemistry, UC Berkeley)
Tenure: 2017
Current positions: MD candidate
 Sophie Shen (College of Chemistry, UC Berkeley)
Sophie Shen (College of Chemistry, UC Berkeley)
Tenure: 2017 – 2018
Publications: 2 total
Current position: PharmD candidate, UCSF School of Pharmacy
 Julia K. Lee (College of Chemistry, UC Berkeley)
Julia K. Lee (College of Chemistry, UC Berkeley)
Tenure: 2017- 2018
Publications: 1 total
Current position: PharmD candidate, UCSF School of Pharmacy
 Suzanna Tom (College of Chemistry, UC Berkeley)
Suzanna Tom (College of Chemistry, UC Berkeley)
Tenure: 2018
Current position: MD candidate, University of South Florida, FL
 Kayla Panora (College of Chemistry, UC Berkeley)
Kayla Panora (College of Chemistry, UC Berkeley)
Tenure: 2018
Current position: Regulatory affairs specialist, Abbott Labs, CA
 Mariaelena Nabors (Amgen Summer Scholars Program, University of Kansas)
Mariaelena Nabors (Amgen Summer Scholars Program, University of Kansas)
Tenure: 2019
Current position: PhD candidate (Biomedical and Biological Sciences), Jen Jen Yeh lab, UNC Chapel Hill, NC
 Nicole Nagayama (St. Mary’s College, CA)
Nicole Nagayama (St. Mary’s College, CA)
Tenure: 2020
Current position: Undergraduate, St. Mary’s College, CA
 Ningjing (Nora) Zhang
Ningjing (Nora) Zhang
Former Staff:
 Garima Arvikar, PhD, (Associate Specialist)
Garima Arvikar, PhD, (Associate Specialist)
 Christopher Drake, PhD (Assistant Researcher)
Christopher Drake, PhD (Assistant Researcher)
Tenure: 2014 – 2016
Publications: 2 total (1 first and co-corresponding author)
Current position: Director of Radiopharmaceutical Contract Manufacturing, SOFIEBIO, Culver City, CA
 Nima Hooshdaran (Staff Research Associate)
Nima Hooshdaran (Staff Research Associate)
 Lisa Wu, PhD (Assistant Specialist)
Lisa Wu, PhD (Assistant Specialist)
Tenure: 2015 – 2016
Current position: Lecturer, Department of Chemistry, San Francisco State University, CA
 Leila Ranis, MS (Staff Research Associate)
Leila Ranis, MS (Staff Research Associate)
Tenure: 2016 – 2017
Current position: Senior scientist, Bio-Rad Laboratories, Hercules, CA
 Yung-hua Wang (Junior Specialist)
Yung-hua Wang (Junior Specialist)
Tenure: 2017 – 2021
Publications: 13 total (1 co-first author)
Current position: Assistant specialist, Gladstone Institutes, San Francisco, CA
Former Rotation Students:
Funding
The Evans lab graciously accepts financial support from the following sources:

Research Directions
Chemical strategies to augment the antitumor effects of targeted radiotherapies
Recent FDA approvals (e.g. Lutathera, Pluvicto) and a swell of promising experimental agents in clinic trials underlie a surging enthusiasm for radioligand therapy (RLT) as a treatment modality in many cancers. However, clinical experience also shows that tumor responses to RLTs are often transient and/or variable among patients. Thus, there is a pressing need for improved understanding and new strategies that maximize the therapeutic benefit of RLT for cancer patients.
For the past decade, the nuclear medicine field has prioritized developing low molecular weight small molecule, peptide, or biologic RLTs that rapidly exit the bloodstream, with the objective of minimizing host toxicity. Despite rapid clearance, small RLTs can effectively treat tumors if they potently bind a highly overexpressed tumor protein (e.g. PSMA, SSTR2, FAP, GRPR). However, RLTs targeting endogenous ligand binding pockets on cell surface receptors co-opt a signaling mechanism that is evolutionarily designed to be transient, and thus ligand dissociation and/or receptor degradation limits the lifetime of ligand–receptor interactions. Thus, the current state of the art likely does not fully maximize the antitumor effects of isotopes like Lu-177 and Ac-225, which have half-lives that span many days to even weeks. On this basis, developing new chemical strategies to deliver and retain radioisotopes in tumors is a meritorious goal.
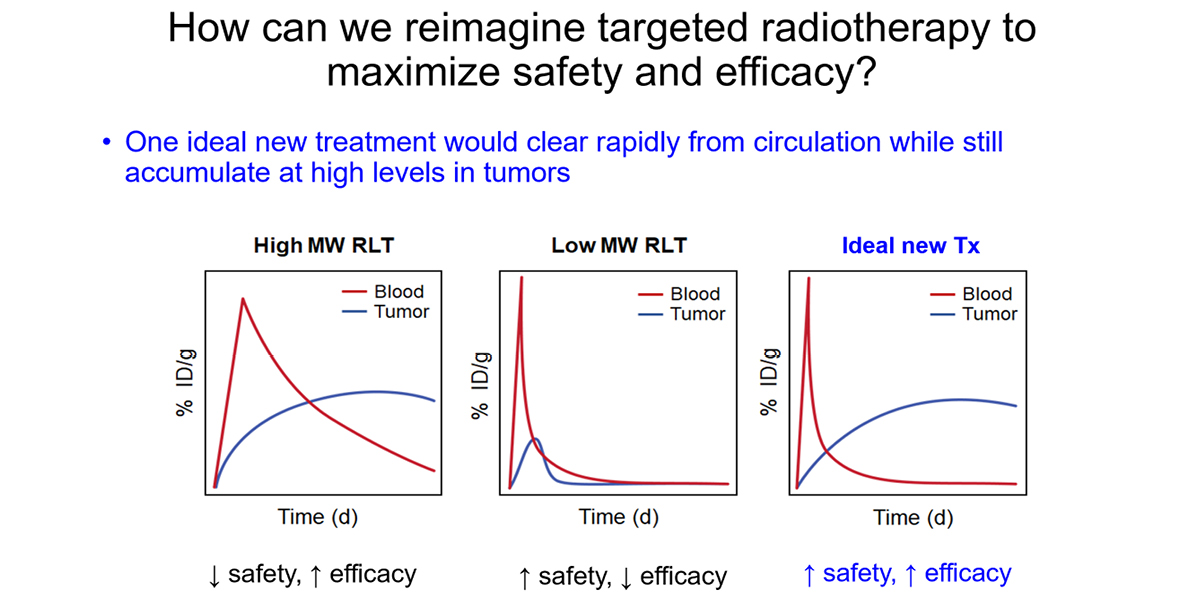
Addressing this challenge is a major emphasis of the lab. Recently, we showed that installing covalency into an RLT can increase tumoral absorbed dose without impacting the overall pharmacokinetic profile of the therapy. Using an anti-HER2 nanobody, we collaborated with Lei Wang’s lab at UCSF to show that installing a lysine reactive unnatural amino acid triggered covalent crosslinking with cell surface HER2 on human xenografts in mice. Crosslinking increased tumoral AUC compared to the noncovalent nanobody by eliminating ligand/receptor dissociation (i.e. koff) thereby extending the retention time of the isotopic payload in tumors. The 225Ac-labeled covalent nanobody more potently suppressed xenograft growth compared to the wild type, non-covalent antibody, as expected. Notably, the PK profile of the mutant nanobody was not significantly impacted by the presence of a mildly reactive electrophile. This is one of several strategies we are pursuing in collaboration with others like Drs. Adam Renslo, Kevan Shokat, Charly Craik, and Jim Wells.
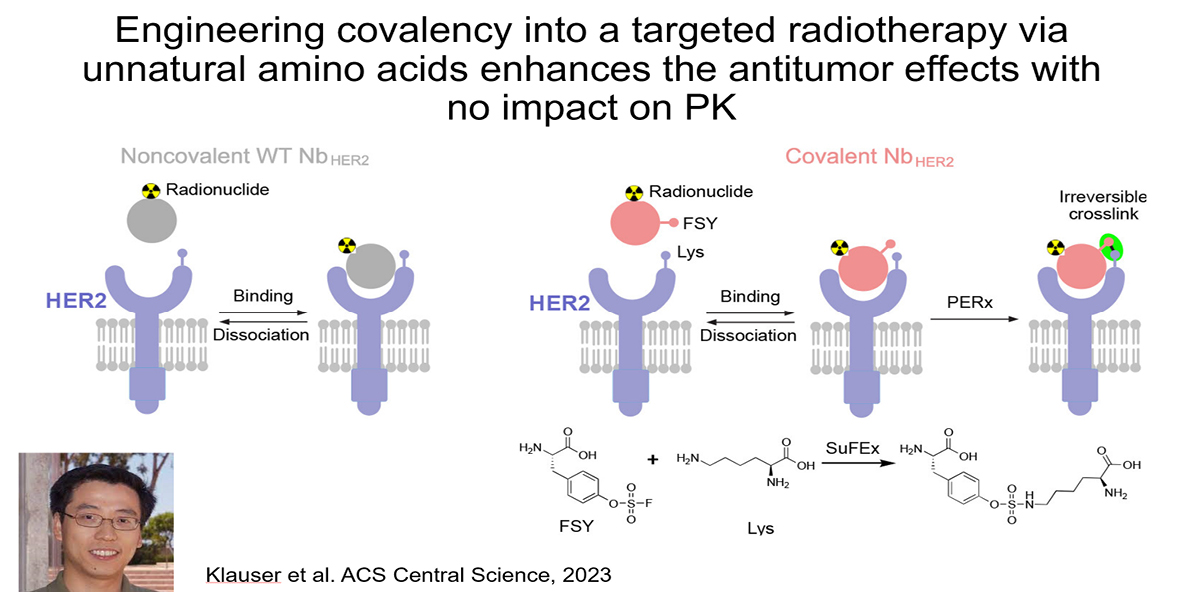
Relevant publications:
- Proteins as Targeted Radionuclide Therapies Enhance Antitumor Effects. ACS Cent Sci. 2023 Jun 28; 9(6):1241-1251.Klauser PC, Chopra S, Cao L, Bobba KN, Yu B, Seo Y, Chan E, Flavell RR, Evans MJ, Wang L. PMID: 37396859; PMCID: PMC10311652.
Studying antitumor immunity with granzyme targeted PET
The recent success of inhibitors against immune checkpoint proteins (e.g. CTLA-4, PD-L1), which are thought in part to stimulate T cell responses against tumors, and chimeric antigen receptor (CAR) T cells has revolutionized cancer therapy. Yet only ~20-30% of patients achieve deep responses, and discerning responders from non-responders is challenging with conventional imaging. Thus, there is an urgent unmet need to develop new imaging biomarkers to distinguish responsive and resistant patients, as well as to detect undesired immune related adverse events.
We hypothesized that an imaging technology capable of selectively measuring the biology utilized by T cells to impart cytotoxicity could predict early tumor responses. Since T cell cytotoxicity against tumor cells is primarily conferred by the pro-apoptotic serine protease granzyme B (GZMB), we have developed a novel probe termed GRIP B that measures the biochemistry of GZMB as it traverses the pericellular space at the immunological synapse. GRIP B is modeled after a “restricted interaction peptide”, a technology that we recently pioneered to enable the first spatiotemporal measurements of endoprotease biochemistry in vivo with PET. Mechanistically, an inactive “pro-form” of radiolabeled GRIP B is administered systemically, whereupon cleavage by GZMB releases a radiolabeled (non-toxic) antimicrobial peptide that spontaneously adopts a helical conformation to immediately associate with nearby phospholipid membranes. Thus, sequestration of the radiotracer by the endoprotease provides a readout of the relative units of enzyme activity within a region of interest. The specificity of GRIP B is driven by the biochemistry of GZMB, which is the only known extracellular protease in humans that can cleave a peptide with an aspartic acid at the P1 site.
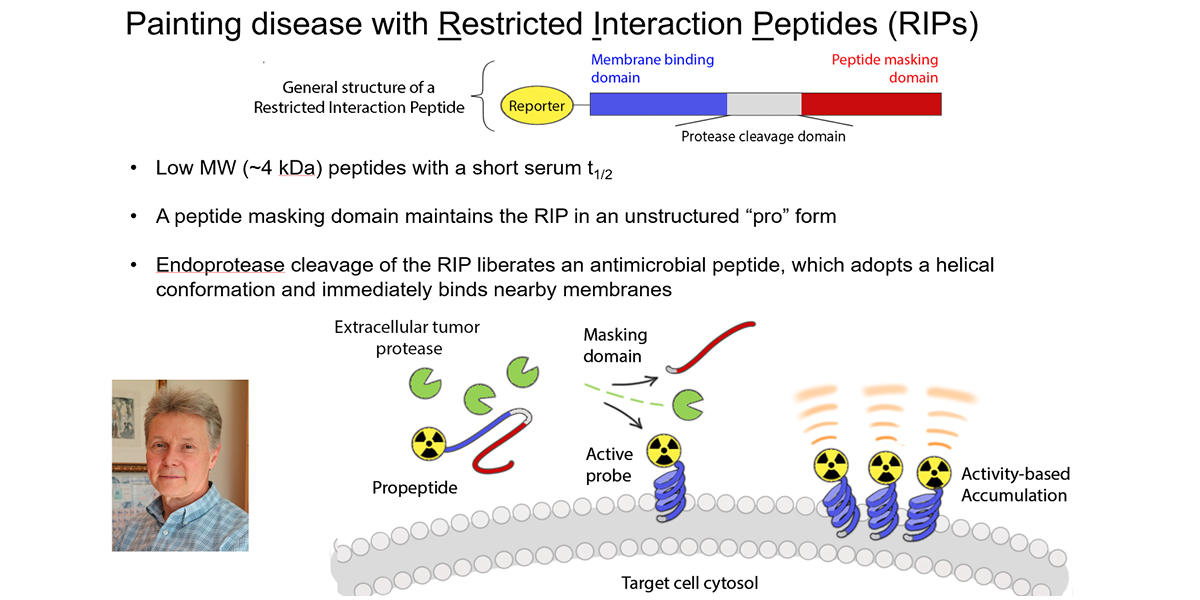
Our preclinical data in mouse cancer models show that 64Cu-labeled GRIP B detects granzyme B from T cells activated with anti-PD-1 and anti-CTLA4 therapies. Provocatively, the radiotracer detects proteolytic activity within tumors, but also among normal tissues (e.g. the spleen) where immune cells would be exposed to systemic immune checkpoint inhibition, which are known to induce immune-mediated toxicities. We confirmed the specificity of 64Cu-GRIP B for GZMB in vivo by conducting imaging studies in tumor bearing mice with germline GZMB knockout, and by applying an uncleavable, D amino acid adduct of 64Cu-GRIP B. Lastly, we have shown that post treatment tumoral uptake of 64Cu-GRIP B is inversely correlated with volumetric tumor responses to checkpoint inhibitors in the wild type, but not GZMB-/- mouse background. We recently opened a clinical trial at UCSF to test 64Cu-GRIP B as a biomarker of response to immunotherapies (NCT05888532).
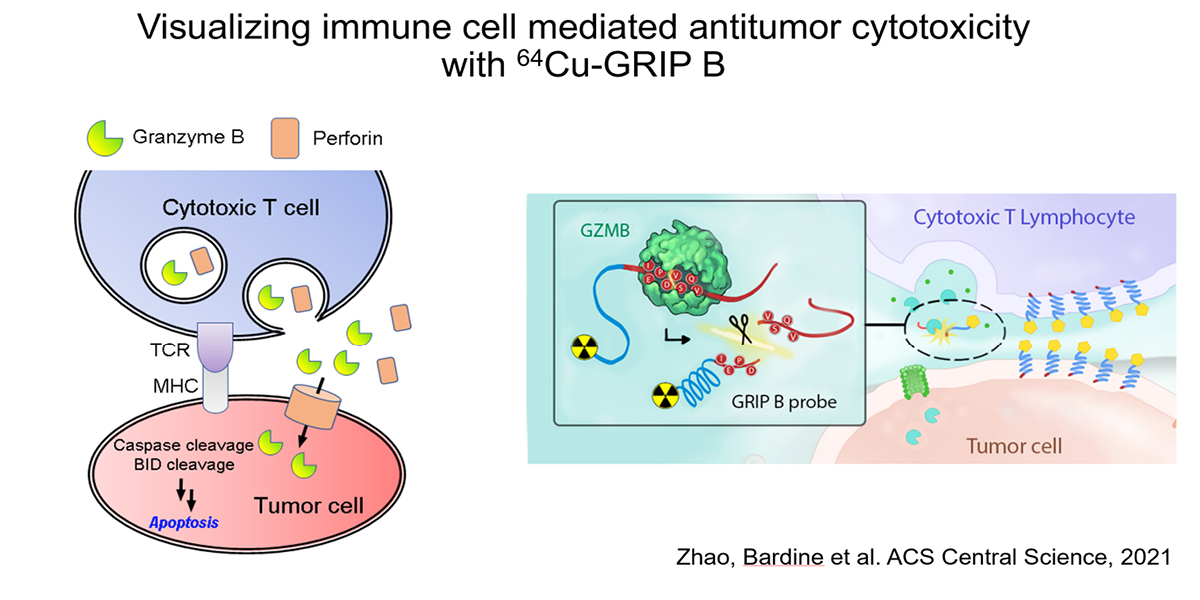
Relevant publications:
- In Vivo Measurement of Granzyme Proteolysis from Activated Immune Cells with PET. ACS Cent Sci. 2021 Oct 27; 7(10):1638-1649.Zhao N, Bardine C, Lourenço AL, Wang YH, Huang Y, Cleary SJ, Wilson DM, Oh DY, Fong L, Looney MR, Evans MJ, Craik CS. PMID: 34729407; PMCID: PMC8554823.
Ferronostics: new technology development to measure iron dyshomeostasis with PET to identify diseases susceptible to therapies targeting iron
We have developed new technologies to image iron with positron emission tomography (PET) in an oxidation state specific fashion and conducted radiomic studies in animals and humans to better understand how to exploit iron dyshomeostasis for the detection or treatment of human diseases. We are recognized for the discovery that mTORC1 and/or MYC are primary drivers of cellular uptake of ferric iron via transferrin in tumors and neoplastic disorders like LAM. Achieving this in mice was enable by new technology development (e.g. 89Zr-labeled transferrin) to measure ferric iron deposition in the most clinically relevant animal models (e.g. GEMMs, PDX’s). I have also led seven clinical trials in patients with cancer or LAM studying ferric iron uptake using 68Ga-citrate PET (68Ga is a ferric iron biomimetic in vivo). Radiomic studies have shown that MYC and/or mTORC1 hyperactivity are the primary drivers of ferric iron uptake, a finding that has since motivated us to test if disorders bearing mutations in these pathways are susceptible to therapies that deplete or further exacerbate iron dyshomeostasis to promote “ferroptosis”. To further enable these studies, we recently developed the first chemical sensor (18F-TRX) to detect the cytosolic pool of bioactive ferrous iron (the so called “labile iron pool”) with PET. This radiotracer has enabled unprecedented measurements of cellular LIP in living subjects, which is essential to our understanding of iron regulation as LIP cannot be measured with conventional analytical techniques that require disrupting the native cellular environment (e.g. ICP-MS). Monitoring LIP in vivo with PET will help us predict if LIP concentration is sufficiently augmented to target therapeutically, or if treatments designed to induce ferroptosis are efficacious. In summary, my lab is internationally recognized as a leader in the development of theranostic strategies to target iron dyshomeostasis.
Relevant Publications
- Holland JP, Evans MJ, Rice SL, Wongvipat J, Sawyers CL, Lewis JS. Annotating MYC status with 89Zr-transferrin imaging. Nat Med. 2012 Oct; 18(10):1586-91. PMID: 23001181.
- Evans MJ, Holland JP, Rice SL, Doran MG, Cheal SM, Campos C, Carlin SD, Mellinghoff IK, Sawyers CL, Lewis JS. Imaging tumor burden in the brain with 89Zr-transferrin. J Nucl Med. 2013 Jan; 54(1):90-5. PMID: 23236019.
- Doran MG, Carnazza KE, Steckler JM, Spratt DE, Truillet C, Wongvipat J, Sawyers CL, Lewis JS, Evans MJ. Applying 8? Zr-Transferrin To Study the Pharmacology of Inhibitors to BET Bromodomain Containing Proteins. Mol Pharm. 2016 Feb 01; 13(2):683-8. PMID: 26725682.
- Behr SC, Aggarwal R, Seo Y, Aparici CM, Chang E, Gao KT, Tao DH, Small EJ, Evans MJ. A Feasibility Study Showing [68Ga]Citrate PET Detects Prostate Cancer. Mol Imaging Biol. 2016 12; 18(6):946-951. PMID: 27184068.
- Truillet C, Cunningham JT, Parker MFL, Huynh LT, Conn CS, Ruggero D, Lewis JS, Evans MJ. Noninvasive Measurement of mTORC1 Signaling with 89Zr-Transferrin. Clin Cancer Res. 2017 Jun 15; 23(12):3045-3052. PMID: 28007777.
- Mari Aparici C, Behr SC, Seo Y, Kelley RK, Corvera C, Gao KT, Aggarwal R, Evans MJ. Imaging Hepatocellular Carcinoma With 68Ga-Citrate PET: First Clinical Experience. Mol Imaging. 2017 Jan-Dec; 16:1536012117723256. PMID: 28893116.
- Aggarwal R, Behr SC, Paris PL, Truillet C, Parker MFL, Huynh LT, Wei J, Hann B, Youngren J, Huang J, Premasekharan G, Ranatunga N, Chang E, Gao KT, Ryan CJ, Small EJ, Evans MJ. Real-Time Transferrin-Based PET Detects MYC-Positive Prostate Cancer. Mol Cancer Res. 2017 09; 15(9):1221-1229. PMID: 28592703.
- Behr SC, Villanueva-Meyer JE, Li Y, Wang YH, Wei J, Moroz A, Lee JK, Hsiao JC, Gao KT, Ma W, Cha S, Wilson DM, Seo Y, Nelson SJ, Chang SM, Evans MJ. Targeting iron metabolism in high-grade glioma with 68Ga-citrate PET/MR. JCI Insight. 2018 11 02; 3(21). PMID: 30385712.
- Muir RK, Zhao N, Wei J, Wang YH, Moroz A, Huang Y, Chen YC, Sriram R, Kurhanewicz J, Ruggero D, Renslo AR, Evans MJ. Measuring Dynamic Changes in the Labile Iron Pool in Vivo with a Reactivity-Based Probe for Positron Emission Tomography. ACS Cent Sci. 2019 Apr 24; 5(4):727-736. PMID: 31041393.
- Elevated labile iron in castration-resistant prostate cancer is targetable with ferrous iron-activatable antiandrogen therapy. Eur J Med Chem. 2023 Mar 05; 249:115110.Gonciarz RL, Sakhamuri S, Hooshdaran N, Kumar G, Kim H, Evans MJ, Renslo AR. PMID: 36708680; PMCID: PMC10210592.
- Ferrous iron-activatable drug conjugate achieves potent MAPK blockade in KRAS-driven tumors. J Exp Med. 2022 04 04; 219(4).Jiang H, Muir RK, Gonciarz RL, Olshen AB, Yeh I, Hann BC, Zhao N, Wang YH, Behr SC, Korkola JE, Evans MJ, Collisson EA, Renslo AR. PMID: 35262628; PMCID: PMC8916116.
Applying cell surface proteomics and antibody engineering to develop new technologies for cancer "theranostics"
The recent FDA approvals of Lutathera and Pluvicto, multiple billion dollar acquisitions of radiopharmaceutical companies (e.g. Rayzebio, Point Biopharma, Fusion Pharmaceuticals), and the swell of promising drug candidates in development, fully underscore the surging enthusiasm for targeted radiotherapy as a cancer treatment strategy. With this renaissance comes the urgent need to identify cancer specific proteins to avoid the toxicities associated with radiopharmaceuticals whose targets are expressed in normal and tumor tissues.
In collaboration with the Wells lab at UCSF (https://wellslab.ucsf.edu/), we are addressing thsi unmet need by applying proteomics to identify proteins on the cancer cell surface that are strongly induced by undruggable oncogenes (e.g. RAS, transcription factors). Because we are focusing on the surface-ome, antibodies can be rapidly evolved through phage display to bind highly upregulated proteins, and of course functionalized with radioisotopes for imaging or endoradiotherapy.
Following on a recent manuscript describing how mutant KRAS remodels the cancer cell surface-ome, we have shown for the first time that antibodies targeting the ectodomain of CUB domain containing protein 1 can be labeled with radioisotopes to visualize (e.g. Zr-89) and treat (Lu-177, Ac-225) RAS driven cancers. CDCP1 is broadly upregulated in cancer, so the technologies and proof of concept treatment data may have therapeutic applications beyond RAS driven cancers.
Moving forward, we are continuing to collaborate closely with the Wells lab to develop several new proteomics data sets to identify cell surface proteins uprregulated by other "undruggable" or poorly druggable oncogenic drivers of cancer. For example, we recently disclosed how mTORC1 activity remodels the cell surface-ome, which may lead to more ablative therapeutic strategies compared to treatment with cytostatic rapalogues.
Relevant publications:
- Martinko AJ, Truillet C, Julien O, Diaz JE, Horlbeck MA, Whiteley G, Blonder J, Weissman JS, Bandyopadhyay S, Evans MJ, Wells JA. Targeting RAS-driven human cancer cells with antibodies to upregulated and essential cell-surface proteins. Elife. 2018 01 23; 7. PMID: 29359686.
- Moroz A, Wang YH, Sharib JM, Wei J, Zhao N, Huang Y, Chen Z, Martinko AJ, Zhou J, Lim SA, Zhang LH, Seo Y, Carlin S, Leung KK, Collisson EA, Kirkwood KS, Wells JA, Evans MJ. Theranostic targeting of CUB domain containing protein 1 (CDCP1) in pancreatic cancer. Clin Cancer Res. 2020 Apr 27. PMID: 32341034.
- Wei J, Leung K, Truillet C, Ruggero D, Wells JA, Evans MJ. Profiling the Surfaceome Identifies Therapeutic Targets for Cells with Hyperactive mTORC1 Signaling. Mol Cell Proteomics. 2020 02; 19(2):294-307. PMID: 31792071.
- Theranostic Targeting of CUB Domain-Containing Protein 1 (CDCP1) in Multiple Subtypes of Bladder Cancer. Clin Cancer Res. 2023 04 03; 29(7):1232-1242.Chopra S, Trepka K, Sakhamuri S, Carretero-González A, Zhu J, Egusa E, Zhou J, Leung K, Zhao N, Hooshdaran N, Feng FY, Wells JA, Chou J, Evans MJ. PMID: 36648492; PMCID: PMC10073270.
- CUB Domain-Containing Protein 1 (CDCP1) Is a Target for Radioligand Therapy in Castration-Resistant Prostate Cancer, including PSMA Null Disease. Clin Cancer Res. 2022 07 15; 28(14):3066-3075.Zhao N, Chopra S, Trepka K, Wang YH, Sakhamuri S, Hooshdaran N, Kim H, Zhou J, Lim SA, Leung KK, Egusa EA, Zhu J, Zhang L, Foye A, Sriram R, Chan E, Seo Y, Feng FY, Small EJ, Chou J, Wells JA, Aggarwal R, Evans MJ. PMID: 35604681; PMCID: PMC9288514.
Developing new radioligands to measure glucocorticoid receptor expression
with PET
The glucocorticoid receptor is a master regulator of normal physiology and its dysregulation is thought to drive the pathophysiology of many diseases, including mood disorders, metabolic diseases, and even cancer. Cell and genetically engineered animal models have been the mainstays for carefully articulating the abovementioned features of GR (patho)biology. Fully understanding GR’s role in human physiology and disease, as well as developing next generation therapeutics to more selectively modulate GR, requires new technologies that can safely probe GR signaling in living subjects. The lack of non-invasive biomarkers to interrogate GR signaling in humans has left the field to perform very suboptimal assays, for instance, analysis of GR and target gene expression levels from tissues collected at autopsy.
To better interrogate GR signaling dynamics in humans, we have developed a library of radiolabeled corticosteroids and synthetic agonists to measure GR expression levels in vivo. These tools have enabled an unprecedented view of GR expression dynamics to reveal aspects of GR biology that are only occurring in vivo. Moreover, we have demonstrated that GR PET can be used to reveal unexpected tissue specific drug-GR interactions, which establishes an entirely new paradigm for the development of selective GR modulators-a major unmet clinical need. Lastly, I supervise an ongoing clinical trial to translate one radioligand, 11C-YJH08, into humans to make the first non-invasive measurements of GR expression levels in healthy subjects and patients with mood disorders or prostate cancer.
Relevant Publications:
- Huang Y, Zhao N, Wang YH, Truillet C, Wei J, Blecha JE, VanBrocklin HF, Seo Y, Sayeed M, Feldman BJ, Aggarwal R, Behr SC, Shao H, Wilson DM, Villanueva-Meyer JE, Gestwicki JE, Evans MJ. A novel radioligand reveals tissue specific pharmacological modulation of glucocorticoid receptor expression with positron emission tomography. ACS Chem Biol. 2020 Apr 07. PMID: 32255605.
- Truillet C, Parker MFL, Huynh LT, Wei J, Jami KM, Wang YH, Shen YS, Sriram R, Wilson DM, Kurhanewicz J, Evans MJ. Measuring glucocorticoid receptor expression in vivo with PET. Oncotarget. 2018 Apr 17; 9(29):20399-20408. PMID: 29755660.
- In Vivo Profiling with 18F-YJH08 Reveals Diverse Tissue Patterns of Antagonist/Glucocorticoid Receptor Interactions. Mol Pharm. 2022 02 07; 19(2):704-709.Kim H, Huang Y, Zhao N, Wang YH, Sakhamuri S, Chopra S, Hooshdaran N, Sriram R, Aggarwal R, Evans MJ. PMID: 35049307.
- The Synthesis and Structural Requirements for Measuring Glucocorticoid Receptor Expression In Vivo with (±)-11C-YJH08 PET. J Nucl Med. 2021 05 10; 62(5):723-731.Huang Y, Zhao N, Wang YH, Truillet C, Wei J, Parker MFL, Blecha JE, Drake CR, VanBrocklin HF, Garrido-Ruiz D, Jacobson MP, Aggarwal R, Behr SC, Flavell RR, Wilson DM, Seo Y, Evans MJ. PMID: 32887758; PMCID: PMC8844266.
- Research Directory
- Abdominal and Pelvic MRI
- Arthritis Imaging Lab (Li Lab)
- Baby Brain Research Group
- Biomagnetic Imaging Laboratory
- Biomechanics & Musculoskeletal Imaging Lab
- Bone Quality Research Lab
- BrainChange Study
- Breast Imaging Research Group
- Breast and Bone Density Group
- Cardiothoracic Imaging Imaging Research
- Center for Molecular and Functional Imaging (CMFI)
- Contrast Material and CT Translational Research Lab
- Evans Lab
- Focused Ultrasound Lab
- High Field MRI Center
- Imaging Research for Neurodevelopment
- Interventional Radiology Research Lab
- Larson Group
- Lupo Lab
- Multimodal Metabolic Brain Imaging
- Musculoskeletal Quantitative Imaging Research
- Neural Connectivity Lab
- Osteoid Osteomas HIFU Clinical Trial
- PET/SPECT Radiochemistry
- PSMA PET Scan
- Physics Research Laboratory
- Program for Molecular Imaging and Targeted Therapy
- Prostate Cancer Imaging Lab (Kurhanewicz)
- Quantitative Biomarkers & AI in MSK Imaging
- Research
- Sarah J. Nelson Lab
- Surbeck Laboratory
- Translational Metabolic Imaging Lab
- Vascular Imaging Research Center
- Viswanath Lab
- Wilson Lab
- Imaging Research Symposium
- Research Conference
- UCSF Radiology at RSNA
- Core Services
Evans Lab
Genentech Hall, UCSF Mission Bay campus
600 16th Street, N552
San Francisco, CA 94158

PI: Michael Evans, PhD
Tel: (415) 353-3442
Fax: (415) 353-9425

