Prostate Specific Membrane Antigen (PSMA) PET Imaging for Prostate Cancer
Offering breakthrough diagnostics for prostate cancer treatment
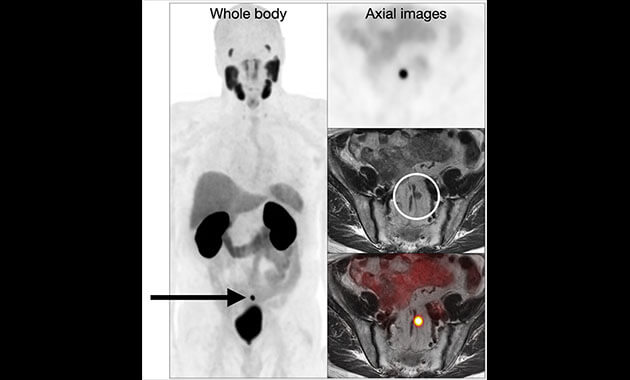
Prostate cancer is one of the most common cancers in men. The new prostate-specific membrane antigen (PSMA) PET imaging will significantly improve how prostate cancer is detected and treated. The FDA approved the drug for positron emission tomography (PET) imaging of PSMA-positive lesions in men with prostate cancer. 68Ga-PSMA-11 is a radioactive imaging agent that binds to prostate cancer cells to help localize prostate cancer cells.
What is prostate-specific membrane antigen PET? (PSMA PET Scan)
- New imaging technique for prostate cancer that locates cancer lesions.
- PSMA PET uses a PET-sensitive drug (68Ga-PSMA-11) that is FDA approved.
- 68Ga-PSMA-11 is a radioactive imaging agent that binds to prostate cancer cells to help localize prostate cancer cells.
How does PSMA PET work?
- Radioactive tracer drug (68Ga-PSMA-11) is injected and attaches to PSMA proteins (prostate cancer tumors overexpress this protein).
- The PET scan detects the concentrated PSMA tracer, pinpointing these tumors for more effective treatment.
How is PSMA PET imaging different from current prostate cancer imaging?
- Current standard technique called fluciclovine PET, involves physicians injecting patients with a synthetic radioactive amino acid.
- PSMA PET imaging is a FDA approved scan with more precise detection of prostate cancer for better treatment planning and targeted care.
- More effective in pinpointing and eliminating tumors not only in the prostate but also throughout the pelvis and the body in cases where the tumors have migrated.
- Imaging with PSMA PET was able to detect significantly more prostate lesions than fluciclovine PET in men who had undergone a radical prostatectomy but had experienced a recurrence of their cancer.
What are the benefits of PSMA PET treatment at UCSF?
- FDA approved imaging technique for prostate cancer.
- The PSMA PET scan can identifiy cancer that is often missed by current standard-of-care imaging techniques.
- The PSMA tracer can also be used in conjunction with CT or MRI scans.
- UCSF is only one of two medical centers in the U.S. that offers the FDA approved PSMA PET.
- PSMA PET is more effective and precise for localizing mestatic prostate cancer.
- UCSF researchers, along with colleagues at UCLA, studied PSMA PET for several years to better precisely locate prostate cancer.
- PSMA PET works using a radioactive tracer, called 68Ga-PSMA-11, which is manufactured on site at UCSF.
Who should consider PSMA PET?
- Men who are initially diagnosed with prostate cancer with risk for metastic disease.
- Men who were previously treated (typically with radiation therapy or prostatectomy), but who have developed biochemical recurrence as shown by a rising PSA (prostate specific antigen).
How to get a PSMA PET Scan?
- PSMA PET scans are offered at UCSF Radiology China Basin location in the San Francisco Bay area.
- For UCSF patients, please reach out to Radiology Scheduling (415) 353-3900 directly to schedule your PSMA scan.
- For non-UCSF facilities referring patients that are new to UCSF, please fax the following to (415) 353-7299 for patient registration to be completed prior to scheduling:
- Patient Exam Order
- Demographics
- Insurance Information and Authorization (if needed)
How to prepare for a PSMA PET Scan?
- See how to prepare for a PET/CT Scan.
What happens during the PSMA PET Scan?
- The radioactive tracer drug (68Ga-PSMA-11) is injected.
- The patient waits one hour before being scanned.
How long does a PSMA PET Scan take?
- The entire PSMA PET scan procedure takes about 2 hours.
- The PET scan takes about 30 minutes.
Support our research
Make a Gift