7T and 3T Whole-Body MRI at Mission Bay
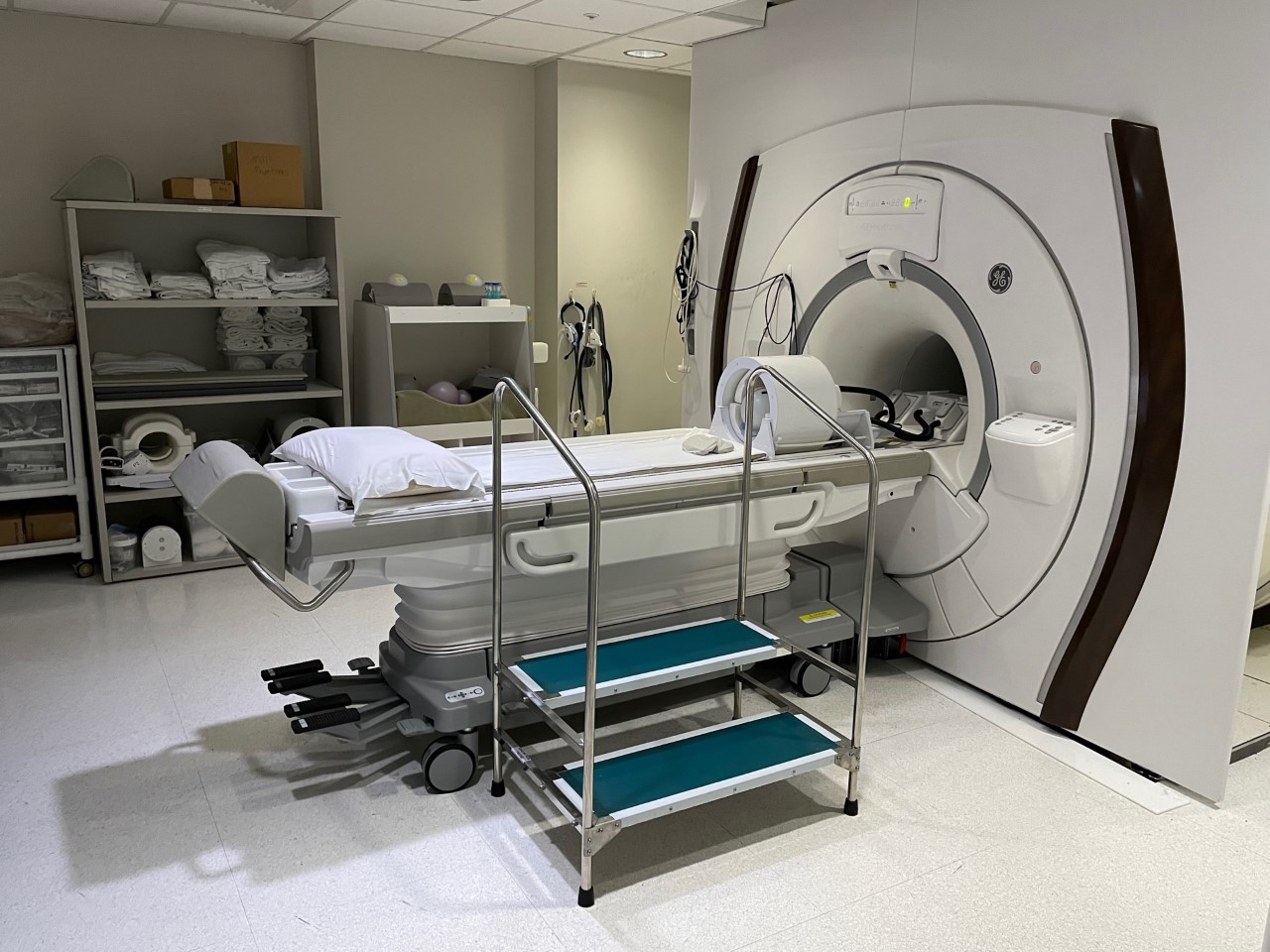 The 7T MR950 scanner has advanced technology from GE Healthcare with improved gradient performance and a 32-channel receive system with multi-nuclear capability. The system fits the FDA designation of a non-significant risk device and uses the same safety criteria as standard clinical MR scanners. The major applications considered so far have focused on neurological diseases such as cancer, neuro-degenerative and psychiatric conditions and musculoskeletal diseases. Dedicated technical support staff and a research nurse are available to manage research subjects and to assist users in application development. Ongoing collaborations include joint projects with the departments of neurology, psychiatry, pediatrics, neurological surgery and orthopedic surgery at UCSF, with other academic institutions and with industry partners.
The 7T MR950 scanner has advanced technology from GE Healthcare with improved gradient performance and a 32-channel receive system with multi-nuclear capability. The system fits the FDA designation of a non-significant risk device and uses the same safety criteria as standard clinical MR scanners. The major applications considered so far have focused on neurological diseases such as cancer, neuro-degenerative and psychiatric conditions and musculoskeletal diseases. Dedicated technical support staff and a research nurse are available to manage research subjects and to assist users in application development. Ongoing collaborations include joint projects with the departments of neurology, psychiatry, pediatrics, neurological surgery and orthopedic surgery at UCSF, with other academic institutions and with industry partners.
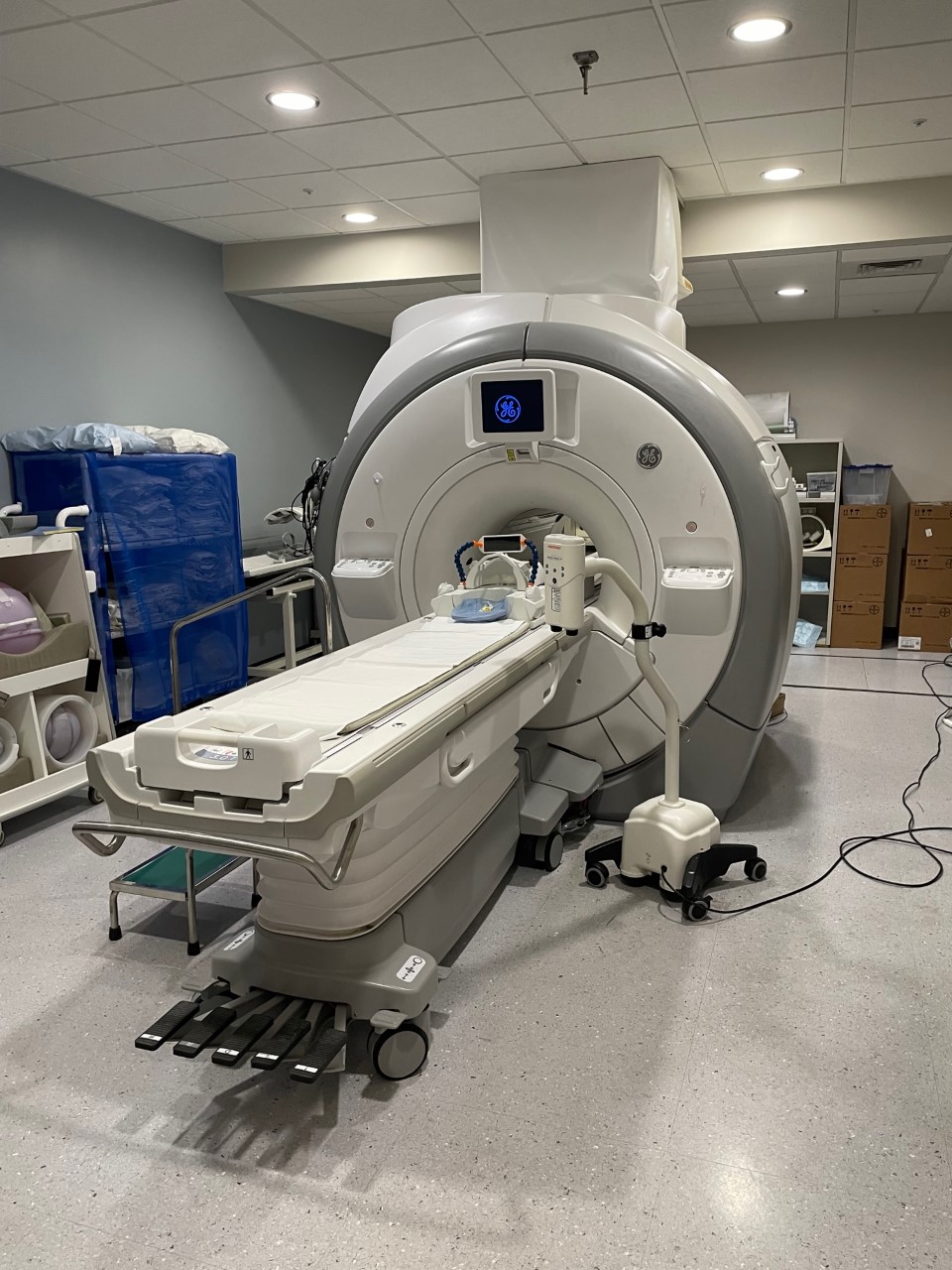 The 3T MRI scanner from GE Healthcare has a 32-channel receiver system, multi-nuclear spectroscopy with 32 channel capability enabling high performance hyperpolarized C-13 studies, high speed / high volume data pipeline, high performance gradients and a wide assortment of specialized MR detectors. In addition to the standard features, it has numerous advanced capabilities developed by UCSF researchers that can be used for the investigation of human disease and monitoring therapeutic interventions in abdominal, musculoskeletal, and neurological systems. Also, hardware for visual and auditory task presentations enable novel functional MRI research studies.
The 3T MRI scanner from GE Healthcare has a 32-channel receiver system, multi-nuclear spectroscopy with 32 channel capability enabling high performance hyperpolarized C-13 studies, high speed / high volume data pipeline, high performance gradients and a wide assortment of specialized MR detectors. In addition to the standard features, it has numerous advanced capabilities developed by UCSF researchers that can be used for the investigation of human disease and monitoring therapeutic interventions in abdominal, musculoskeletal, and neurological systems. Also, hardware for visual and auditory task presentations enable novel functional MRI research studies.

Advanced instrumentation for generating hyperpolarized C-13 imaging agents is also available for use in conjunction with the 3T scanner for studies in humans and model systems. Dedicated support staff assist to users in application development and expert imaging scientists provide advice on data acquisition and analysis procedures. Research collaborations include multiple UCSF departments and industry, as well as numerous other academic research institutions.
