Kazim Narsinh, MD, on Drug Delivery Across the Blood Brain Barrier
 In “Strategies to Improve Drug Delivery Across the Blood-Brain Barrier for Glioblastoma,” UCSF’s Kazim Narsinh, MD, John de Groot, MD, and collaborators in UCSF’s Helen Diller Cancer Center discussed the challenges of treating glioblastoma, with an emphasis on novel drugs and alternative methods of drug delivery. It also covers the ongoing challenges of drug design, positing combination therapy and novel trial designs with early integration of on-treatment tumor tissue analysis and imaging biomarkers as optimal strategies for early trial designs moving forward.
In “Strategies to Improve Drug Delivery Across the Blood-Brain Barrier for Glioblastoma,” UCSF’s Kazim Narsinh, MD, John de Groot, MD, and collaborators in UCSF’s Helen Diller Cancer Center discussed the challenges of treating glioblastoma, with an emphasis on novel drugs and alternative methods of drug delivery. It also covers the ongoing challenges of drug design, positing combination therapy and novel trial designs with early integration of on-treatment tumor tissue analysis and imaging biomarkers as optimal strategies for early trial designs moving forward.
Glioblastoma (GBM) is the most common and aggressive malignant brain tumor in adults. Despite some advancements in treatment, the prognosis for GBM remains poor, with a median overall survival of only 6.8%. The current standard treatment for GBM consists of surgery, radiation, and Temozolomide chemotherapy. While this treatment can extend life expectancy, it is not curative.
There are many challenges to treatment as GBM tumors are highly variable, making it difficult to target all cancer cells, the tumors suppress the immune system, and the blood-brain-barrier (BBB) restricts the delivery of drugs to the brain. New approaches to drug delivery are needed to counter these obstacles and Narsinh then elaborates on possibilities.
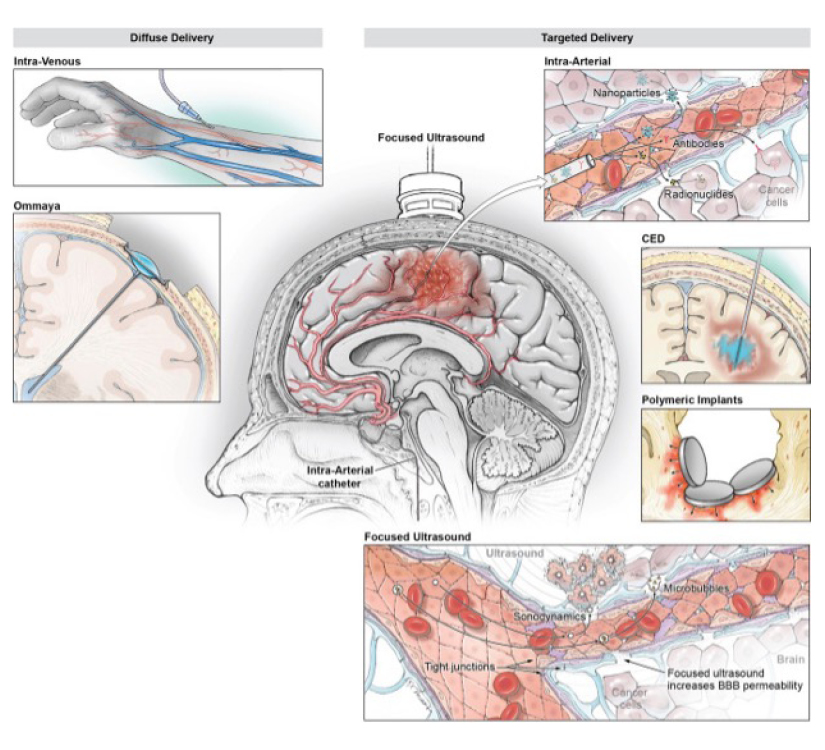 There are treatments in development to overcome those barriers, including novel drug designs such as nanoparticles and antibody–drug conjugates, new methods of drug delivery such as convection-enhanced and intra-arterial delivery, and methods to enhance drug penetration such as blood–brain barrier disruption by MRI-guided focused ultrasound and laser interstitial thermal therapy. MRI-guided focused ultrasound holds tremendous promise as a versatile tool for improving drug delivery and favorably altering the tumor microenvironment.
There are treatments in development to overcome those barriers, including novel drug designs such as nanoparticles and antibody–drug conjugates, new methods of drug delivery such as convection-enhanced and intra-arterial delivery, and methods to enhance drug penetration such as blood–brain barrier disruption by MRI-guided focused ultrasound and laser interstitial thermal therapy. MRI-guided focused ultrasound holds tremendous promise as a versatile tool for improving drug delivery and favorably altering the tumor microenvironment.
Some drugs can be chemically modified to more easily pass the blood-brain barrier, mixing antineoplastic effects with something that increases its solubility or cell permeability, or by exploiting existing mechanisms of barrier entry. A diverse range of nanoparticles can be delivered across the BBB by passive accumulation of plain nanocarriers or by imitating biological entities.
