General
Most pellets and bullets tested for MR compatibility are composed of nonferromagnetic materials. Ammunition that is ferromagnetic tended to be manufactured in foreign countries and or used for military applications. Because pellets, bullets, and shrapnel may be contaminated with ferromagnetic materials, the risk versus benefit of performing an MR procedure in a patient should be carefully considered, as well as whether or not the metallic object is located near a vital anatomic structure. Shrapnel within or adjacent to the globe in the orbit is an absolute contraindication. However, outside of this area most shrapnel is embedded within scar and unlikely to experience significant torque when placed in a magnetic field. Radiographic screening is recommended in patients with a history of gunshot wounds. Plain film radiographs are usually sufficient to confirm that metallic debris is located within subcutaneous, intraosseous or otherwise non-vital tissues; however, suspected proximity to large arteries or viscera should be further investigated with low-dose noncontrast CT when there is ambiguity on plain films.
Please see the Pregnancy section.
Drug infusion pumps are used for automatic delivery of antineoplastic agents or narcotics. While some drug delivery devices are "constant flow" without a motor, infusion pumps have a motor that may have ferromagnetic properties, a magnetic switch, and are programmable by telemetry. Potential MRI issues include:
- Opening of outlet valves resulting in discharge of the entire drug reservoir.
- Temporary or permanent stall of the motor.
- Local tissue heating and increased potential for peripheral nerve stimulation (PNS).
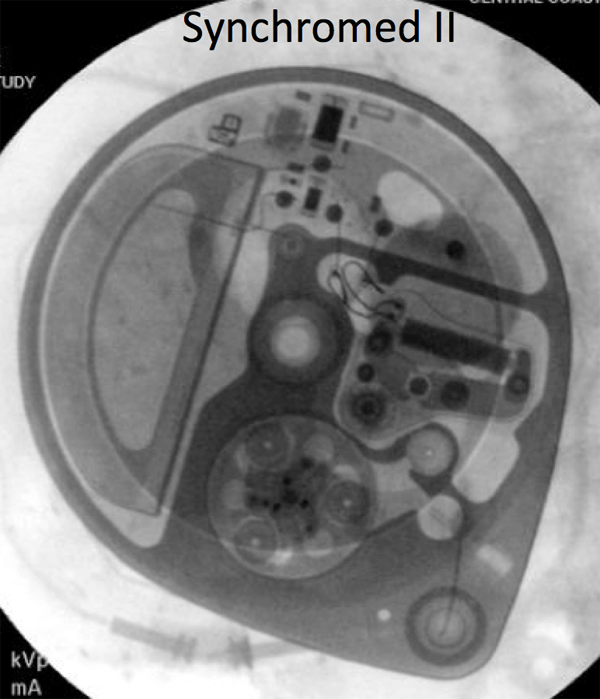
- A policy is being developed with the Pain Management Service to expedite the scanning of patients with Medtronic Synchromed II pumps, which are MRI conditional at 1.5T. The identity of the pump must be documented before the MRI scan, and then confirmed at the time of the scan.The pump program needs to be check after the MRI: for outpatients, this can be done at a pre-scheduled appointment with the managing service; inpatients will need to have the Pain Management team perform this check.
- Constant flow systems (Codman, Medtronic Isomed) are MRI conditional at 1.5T.
- Medallion pumps are investigational at this point, but are reported to be MRI conditional at 1.5 and 3T.
- Codman MedStream pumps are MRI conditional at 1.5 and 3T. Pump program needs to be checked after MRI.
- Flowonix Prometra pumps should never be scanned at UCSF.
If there is any doubt about the suitability of a device for MRI, or about management of a device before and after MRI, contact the Neuromodulation Service ([email protected]). Below are x-ray images of the common Synchromed II pump and the two pumps that should not be imaged without outside consultation and management: Prometra I and II pumps.
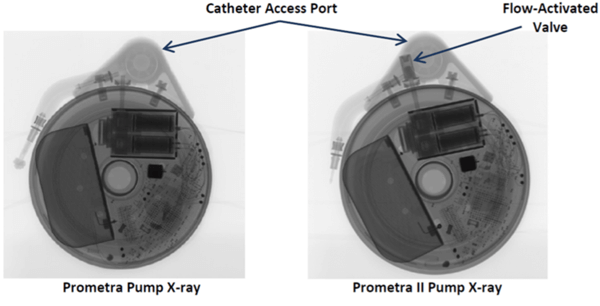
Many epidural and peripheral nerve catheters contain conducting wire that could heat during magnetic resonance imaging (MRI). The Arrow® FlexTip Plus® Epidural catheter is currently being used by neurosurgery and anesthesia here at UCSF and this catheter is rated as MRI conditional with the following imaging limitations:
- Static magnetic field of 3T or less.
- Maximum spatial gradient magnetic field of 720-Gauss/cm or less.
- Use of a transmit/receive head RF coil only.
MRI with this catheter in position is thus safe only for examinations of the head. All other epidural catheters must be researched with the manufacturers for contraindication or conditional imaging guidelines. If there is no information available pertaining to the epidural catheter, it must be removed prior to imaging.
One of the feeding tubes (Mclean-Ring Enteral Feening Tube) used at UCSF is not safe for MRI.
The commonly used Corflo feeding tube (the "yellow" tube, including those with tungsten-weighted tips) is MRI safe at 1.5T and 3T with the stylet removed.
The main issue with imaging spinal fixation hardware (including Harrington rods) is artifact. Heating is also possible. Imaging at 1.5T is preferred with careful attention to patient padding in order to prevent skin-to-skin contact points. Patients should be conscious during imaging to report any unusual heat sensation. If only 3T imaging is available, normal operating mode (SAR of 2 W/kg) should be used.
Neuro
Halo vests pose several risk factors which include deflection and subsequent dislodging of the halo, heating due to RF absorption, electrical current induction within the halo rings, electrical arcing and severe art factual consequences which could render the imaging acquisition useless. Non-ferrous and non-conductive halo vests, which are MR compatible, are commercially available.
There are two general types of neurostimulators:
- Passive receivers: neurostimulators that receive RF energy that is magnetically coupled from an external device by means of a coil placed over the implanted device.
- Hermetically encased pulsed generators: neuro-stimulators that contain a battery and are programmed by an external device to produce the various stimulus parameters.
Exposure of a neurostimulator to the electromagnetic fields used for MR imaging may produce device malfunction and or harm to the patient. Neuro-stimulation systems include the following: Deep Brain Stimulator (DBS), Vagus Nerve Stimulator (VNS), and Spinal Cord Stimulator (SCS). Each device has manufacturer’s conditional imaging guidelines regarding: field strength and RF coil restrictions; SAR limitations; and scan area restrictions.
MRI with these devices should only be performed in strict accordance to manufacturer’s conditional guidelines. If there are abnormal impedances, neurology should add language to acknowledge the impedance issue and their discussion about this with the patient in their notes. A policy is being developed with the UCSF Neurology Service that would allow access to MRI for patients with Medtronic DBS Neurostimulators, which can either be "head-only" or "full-body" eligible:
- Head-only eligible: Model 37602 (Activa SC), Model 7428 (Kinetra), Model 7426 (Soletra).
- Full-body eligible (after 2009): Model 37612 (Activa RC), Model 37603 (Activa SC), Model 37601 (Activa PC).
Scanning of these devices can be performed on the Philips 1.5T (MR4), or on 3T systems that allow monitoring of B1 rms (although not after a complete device has been placed), using the following protocol:
- When an MRI study on a patient with a deep brain stimulator (DBS) is requested, it will be flagged by scheduling and placed in the MRI Manager’s work queue.
- An MR Manager will assess whether a Medtronic DBS Eligibility form has been completed.
- If the elegibility form has been completed, and the device is appropriate, MRI will be scheduled on the Philips 1.5T or an appropriate 3T scanner (go to step 5).
- If there is no completed screening form, an appointment with Monica Volz in Neurology is required (415-353-7382; [email protected]). If the study is scheduled within one week of the Neurology appointment, then skip to step 6.
- Once the MR exam has been scheduled, the Medtronic team will be notified ([email protected]; 415-370-3555) and asked to appear during the patients MR screening to assess DBS function.
- The MRI scanning protocol should be defined by the attending radiologist. If a conforming protocol does not exist, consult with Alastair Martin (415-353-8770), who will help adapt the protocol to comply with Medtronic limits (for full body eligible devices: < 2.0uT B1+rms, maximum active scanning time of 30 minutes within a 90 minute window).
- On the day of the MRI, the Medtronic DBS screening form must either exist in Apex (uploaded by Neurology) or be presented in hardcopy by the patient. The MR tech will review the screening form and make a note in Apex that it was reviewed and everything is in order. If a hardcopy form was presented it will be scanned and uploaded into Apex.
These devices usually have an external electronic component that attaches to electrodes implanted in areas of fractured bones. They are used to enhance and facilitate the rate of bone healing. Review each manufacturer’s guidelines for MRI contraindications or conditional restrictions. More information ay be found at www.mrisafety.com
MR procedures are strictly contraindicated in patients with certain cochlear implants because of the possibility of injuring the patient and/or permanently damaging or altering the function of the cochlear implant devices and/or electrode arrays. However, there have recently been several implants that have received FDA conditional status for MRI. Patients with these cochlear implants can undergo MRI if the following criteria are observed:
- Only approved Baha and MedEL Cochlear Implants (i.e., Sonata, Pulsar, Concert).
- Only at 1.5T.
- Pre-MRI CT of the temporal bone is required for bone thickness.
Some intracranial aneurysm clips are absolute contraindications to MRI. The surgical management of intracranial aneurysms and arteriovenous malformations by the application of aneurysm clips is a well-established procedure. The presence of an aneurysm clip in a patient referred for a MR procedure represents a situation that requires the utmost consideration because of the associated risks.
Certain types of intracranial aneurysm clips (e.g., those made from martensitic stainless steels such as 17-7PH or 405 stainless steel) are prone to torque in a MR produced magnetic field. The displacement of these clips may damage the vessel, resulting in hemorrhage and or death. Intracranial clips made of titanium- or chromium-based alloys are now commonly used and have proved safe for MR.
To minimize the possibility of inadvertently imaging a patient with a magnetically active metallic implant, implanting physicians must provide patients with information about the type and identity of their particular implants, and suggest that the patients carry an alert card, or wear a medical alert bracelet,or necklace, identifying them as having such an implant. In addition, physicians who order MR procedures must carefully screen patients and inform the MR imaging facility of any metallic implants the patient may have and provide written documentation stating the name and model number of the clips.
Traditional EEG electrodes are ferromagnetic and contraindicated for MRI. MRI conditional electrodes have recently been developed and being considered for use at UCSF. EEG electrodes that are MRI conditional afford several advantages:
- Skin breakdown and risk of infection are minimized, as electrodes are not disconnected and then reapplied.
- Patient care delays are minimized because there is no need to wait until after an MRI to apply the electrodes.
- MRI staff does not need to wait for an EEG tech to remove the electrodes.
Examples of the two types of MRI conditional electrodes are pictured below. They disconnect/reconnect quickly, and only the MRI safe portion goes with the patient. An EEG tech will check each patient to make sure only MRI safe leads are going with the patient. An orange coban or a sticker marked with “MRI OK” will be applied to confirm that this check has been completed.
Non-clinical testing of these FDA cleared electrodes has demonstrated that these electrodes can safely remain on a patient during an MRI that contains multiple sequences, as long as a sequence does not exceeed 15 minutes of scan time, and are approved for both 1.5T and 3T. Other stipulations for use are:
- Maximum spatial gradient field of 4,000 gauss/cm (40T/m).
- Maximum whole body SAR of 2W/kg.
- Extension cables much be removed before entering the MRI, as they are unsafe.
Image artifact from the EEG electrodes typical extends no more than 3 mm from the skin attachment at 3T with a gradient echo pulse sequence. More details and safety data can be found at the manufacturers’ sites: iveseegsolutions.com or Rhythmlink.com.
If any questions or concerns arise regarding a particular patient with EEG electrodes, please contact either Dawn Deines at Mission Bay (415-361-8242) or Tom Thompson at Parnassus (415-203-4453).
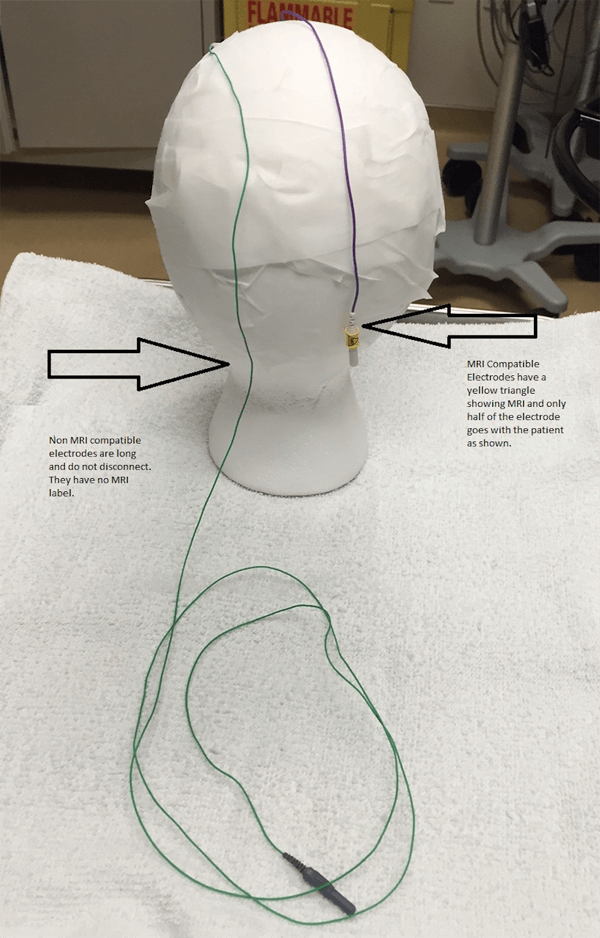
MRI Compatible versus Non-Compatible Electrodes.
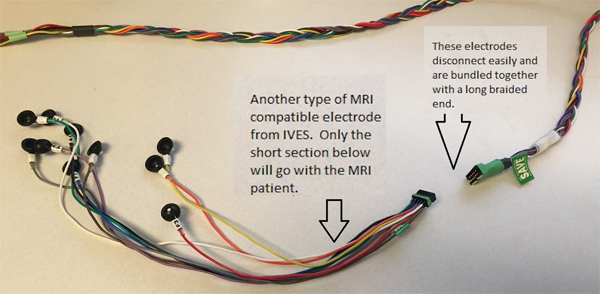
Second MRI Compatible Electrodes.
The Camino Flex Ventricular Catheter is used at UCSF and is MRI safe if imaged in the following way:
- Confirm identity of the catheter.
- 1.5T or 3T is OK.
- Use only the following coils: Transmit / Receive Body Coils; Transmit Body Coils / Body Receive-Coil; Transmit Body Coils / Head Receive Coil.
- Maximum SAR of 2W/kg, or 4W/kg if using first level controlled operating mode.
- Maximum spatial gradient magnetic field of 3500G/cm (35T/m).
- Catheter should have a "coiled" configuration. If this configuration is lost, the MRI scan should be aborted.
Cardiac/Body
Tissue expansion with an inflatable breast implant is a common breast reconstruction technique. These devices typically have an injection site that may be metallic, and even magnetic in some cases. Possible complications with MRI include:
- Artifact that may prevent imaging of adjacent breast tissue.
- Movement that may cause patient discomfort and possibly displacement of the device.
- Burning sensation.
The presence of breast tissue expanders, date of placement and the device manufacturer should be documented in all patients prior to MRI. A chest x-ray may be useful to determine if the device has a metallic component. Imaging at 1.5T may be appropriate in some circumstances if the benefits of imaging clearly outweigh the risks, and the patient is aware of the possible complications and consents to proceed. The following breast tissue expanders are considered MRI unsafe:
- MAGNA-SITE
- Contour Profile Tissue Expander
MRI is not considered hazardous for patients with prosthetic heart valves or annuloplasty rings. The attractive forces exerted on the prostheses are small compared to the force exerted by the contracting heart. There have been no reports of injury from MRI performed in patients with heart valve prostheses or annuloplasty rings.
Please see the dedicated section.
Some penile implants exhibit ferromagnetic qualities and are subject to substantial deflection forces measured when exposed to a 1.5 Tesla magnetic field. Although unlikely to cause significant injury, MRI in patients with these implants would undoubtedly be uncomfortable. For this reason, subjecting a patient with one of these implants to an MR procedure is inadvisable. There are a number of MRI conditional penile implants safe at both 3.0T and at 1.5T. Manufacturer guidelines should be followed for these devices.
A Foley catheter with a temperature probe has a thermistor or thermocouple located on or near the tip of the device and a wire that runs the length of the catheter to a connector that plugs into a temperature monitor. Sometimes an additional external cable is also used, which may or may not be removable. A Foley catheter with a temperature sensor should never be connected to an external cable and/or the temperature monitor during imaging.
Due to the possibility of heating of the wire in the Foley and possible harm to the patient, specific labeling instructions for a given Foley catheter with temperature sensor must be carefully followed. Importantly, not all Foley catheters with temperature sensors are acceptable for patients undergoing MR procedures. The catheters must be FDA approved for use in the MRI scanner. Otherwise, the temperature probe and the corresponding wire must be removed prior to imaging.
The Bardex Latex-Free Temperature-Sensing 400-Series Foley Catheter is commonly used at UCSF and is MRI conditional. For imaging, please:
- Confirm identity of the Foley Catheter.
- Position catheter with temperature sensor in a straight configuration down the center of the MRI table (i.e., aligned with the "z" axis).
- Any removable catheter connector cable should be disconnected.
- 1.5T or 3T is OK with the following stipulations: SAR of 3.5 W/kg (or 3.0 W/kg at 3T) for 15 min of scanning per sequence; spatial gradient magnetic field of 720-Gauss/cm or less.
Two other Foley catheters with temperature probes have been FDA approved for MRI, and may also be used at UCSF:
- Bard Lubricath Temperature-Sensing Foley Catheter, Ref 909116M.
- DeRoyal Foley Catheter with Temperature Sensor.