CEID, Pacemakers, ICDs, Pacing Wires and Loop Recorders
Cardiac Electronic Implated Device (CEID), cardiac pacemaker/implantable cardioverter defibrillator (ICD) in place for more than 6 weeks is strongly preferred. 1.5T scanners are the default option. 3T scanners will only be considered for appropriate MRI conditional devices. Please review cardiac pacemakers/ICD workflow diagram below for overview of how these cases should be handled. Studies will be performed only on MRI conditional devices at San Francisco Veterans Affairs Health Care System. Note that scheduling of outpatients should occur after the "vetting" stage.
6 weeks waiting period facts
- There are some manufacturers that do not require a 6 week waiting period. Referencing current manufacturer's website is recommended.
- There are limited studies showing successful MRI completion in patients without a waiting period, assessment by a MD is required, along with careful attention to limiting patient body/arm movement during positioning for the MRI.
- Pacing leads are electrically conductive, radiofrequency pulses experienced in MRI imaging can induce currents that may result in thermal injuries and severe patient harm.
- Lead heating can be very difficult to predict, as it depends on many factors:
- configuration/orientation
- polarity
- position in the patient AND in the transmit coil
- composition/structure
- shielding/sheathing
- length (resonant heating), resulting in arrythmias
- size and position of patient within the magnet bore
CEID, pacemaker/ICD lead scenarios with guidance
- Temporary epicardial pacing wires - IF cut at the skin, may undergo MRI of any body part at 1.5T or 3T.
- To clarify: these are used temporary epicardial pacing in the setting of cardiac surgery. If the leads cannot be extracted at the end of the procedure, they are left in place and cut at the skin.
- Temporary transvenous pacing leads - are an absolute contraindication to MRI due to increased heating risk (20°C/68°F) and increased risk of current induction.
- Fractured Leads - may be considered according to the non-conditional CEID protocol @ 1.5T, radiologist assessment for indication, risk vs benefit discussion with patient and signed consent.
- Abandoned, in situ, permanent intracardiac pacing leads - may be considered MR Conditional @ 1.5T, using the Non-MRI-Conditional CEID. Radiologist assessment for indication, risk vs benefit discussion with patient and signed consent.
- Abandoned, in situ, epicardial leads - seen infrequently in adults but are common in pediatrics are treated the same as intracardiac leads that are abandoned
- Image artifacts - are a risk when the lead is within imaging area.
- CEID images with lead types:
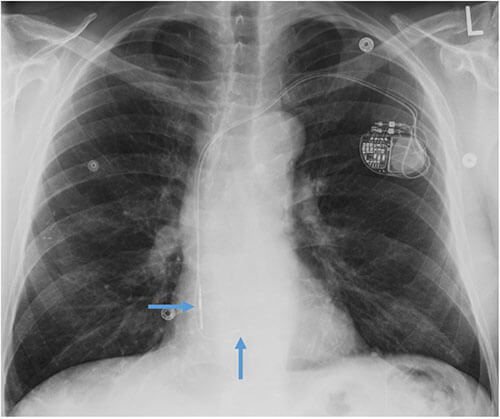 Pacemaker with transvenous leads
Pacemaker with transvenous leads
 Pacemaker with epicardial leads
Pacemaker with epicardial leads
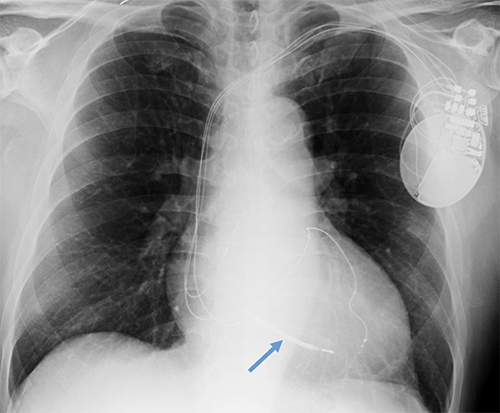 Defibrillator lead
Defibrillator lead
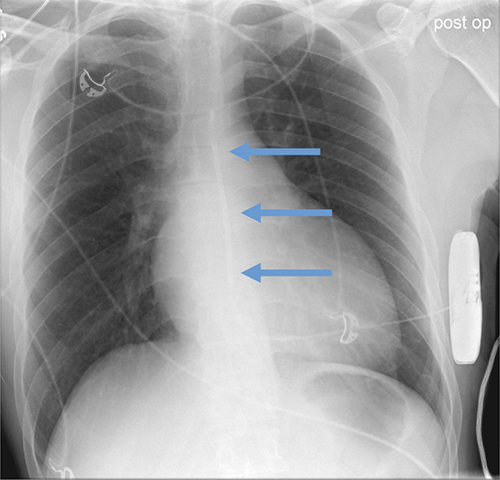 Subcutaneous defibrillator
Subcutaneous defibrillator
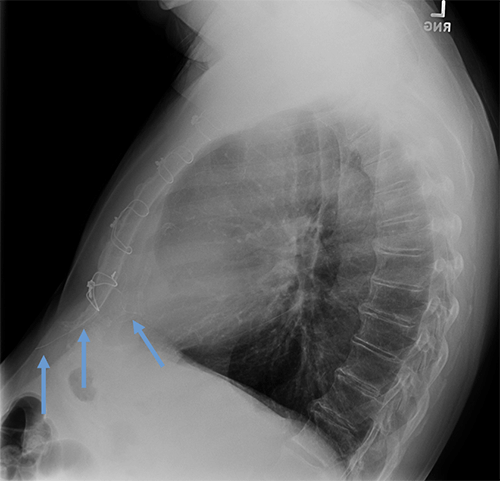 Cut epicardial wires (often hard to see)
Cut epicardial wires (often hard to see)
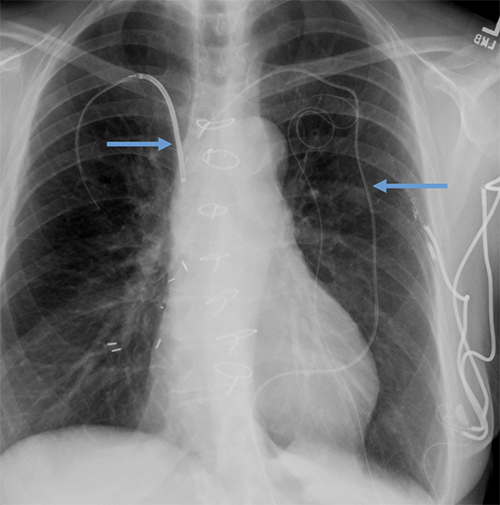 Abandoned leads
Abandoned leads
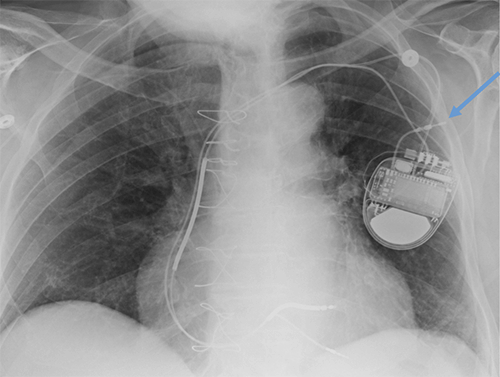 Abanded leads plus generator
Abanded leads plus generator
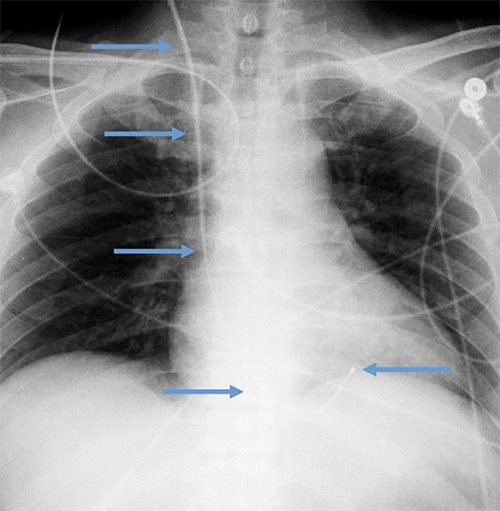 Temporary transvenous lead
Temporary transvenous lead
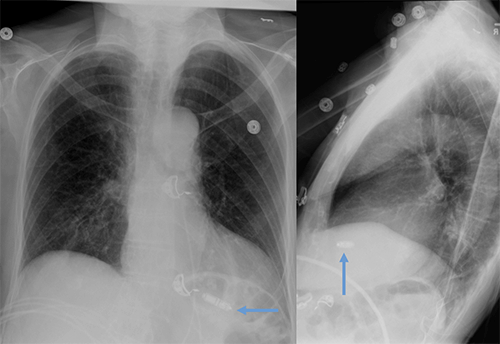 Leadless pacemaker
Leadless pacemaker
Abandoned, in situ intracardiac and epicardial leads
At UCSF, we perform Cardiovascular Magnetic Resonance Imaging in patients with abandoned pacing leads according to our institutional non-MRI-conditional protocol following the same safety protocols as patients with leads attached to generators. This includes static field strength of 1.5T and normal operating mode - ex: SAR (≤1.5 or ≤ 2 W/kg) and dB/dt limitations (<200T/m/s). Patients with abandoned leads/fragments but without a generator in place do not require involvement by electrophysiology. Radiologist assessment for indication, risk vs benefit discussion with patient, and signed consent.
Pacemakers/ICDs
Newly implanted pacemakers and implanted cardioverter/defibrillators are considered MR conditional. However there are approximately 6 million patients with legacy devices, and >50% of these patients will have a clinical indication for MRI during the lifetime of the device.
Potential risks of MR imaging in patients with implanted cardiac devices include movement and/or vibration of the generator and leads, temporary or permanent malfunction of the device, and excessive heating or induced currents in the leads. However, a large body of evidence has demonstrated a low overall rate of complications. Currently there is broad international agreement that both MR conditional and non-MRI-conditional cardiac electronic implanted devices can be safety imaged, including statements from the following groups:
- Heart Rhythm Society
- American Heart Association
- American College of Radiology
- Canadian Heart Rhythm Society
- German Cardiac Society
- British Cardiovascular Society
Specific guidelines for patients with pacemakers/ICDs
The CEID/pacemaker/ICD in place for minimum of 6 weeks is strongly preferred.
Monitoring
Patient monitoring will be performed by a provider with advanced cardiac life support (ACLS) training and MRI safety training for all CEIDs/pacemakers/ICDs.
Continuous visual and voice contact must be maintained with the patient.
In the event that a patient is unresponsive or MRI is requested with anesthesia, radiology attending approval/assessment is required and the exam will only be performed in patients with MR conditional devices.
Exam scheduling
Patients should be scanned during normal workday hours (8am to 5pm) on select 1.5T scanners. 3T will only be considered for appropriate MRI conditional devices.
Device vetting
The device will enter the implant queue for identification and vetting. Verify that documentation of model, manufacturer, lead information is accurate and list most current system components, including revisions, generator replacement.
Expedited approval is sometimes possible if:
- an MRI conditional device is documented in the electronic medical record; and
- an established, modified MRI scan protocol appropriate for cardiac pacemakers/ICDs will be used.
To facilitate this, the specific device in question and MRI scan protocol should be clearly stated in the MRI requisition. Note that established scan protocols are in place for:
- Brain
- Cardiac
- Abdomen
- Knee
- Spine following the manufacturer's guidelines regarding MRI protocol restrictions.
MRI conditional versus non-MRI-conditional status
MRI conditional versus non-MRI-conditional status of the generator, leads, and combination thereof should be determined by UCSF Implant vetting team, principal/senior MR Technologist, MR Technologist Supervisor or MRI MD.
- If the entire CEID system is deemed MR conditional:
- The manufacturer MRI safety labelling will be followed including:
- Static field strength (1.5T vs. 3T)
- Maximum spatial field gradient
- Slew rate
- Specific absorption rate (SAR) limits (most commonly ≤2 W/kg)
- Anatomic location of isocenter
- Scan duration
- Coil restrictions
- Normal operating mode versus first-level controlled mode
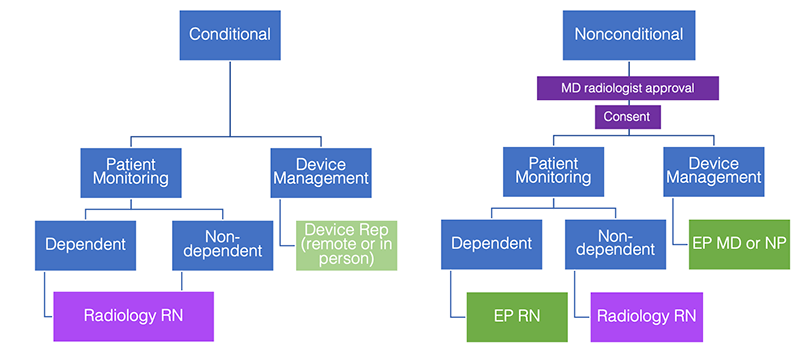
Workflow for MRI conditional cardiac pacemakers/ICDs
- Prior to scheduling, device and leads identified and assessed for conditional/non-MRI-conditional status by any of these roles: UCSF Implant vetting team, principal/senior MR Technologist, MR Technologist Supervisor or MRI MD.
- No radiology MD approval is required if scanned per manufacturer recommendations.
- Device rep (remote or in person) will interrogate and reprogram device at the time of scan.
- Monitoring will be performed by a trained radiology nurse for both pacemaker dependent and nondependent patients.
- Device rep (remote or in person) verifies pacemaker function following completion of the exam.
If any issue arises (i.e., pain, discomfort, significant change in heart rate), the patient will be removed from the MRI scanner and the EP consult fellow will be called at 443-UCEP. If the patient is unresponsive or unstable, a code will be called. An external defibrillator should be easily accessible.
If the system is non-MRI-conditional, the following safety limitations will be implemented:
- MD radiologist approval for appropriateness to proceed
- Risk vs Benefit discussion with signed consent
- Minimum number of MRI sequences performed to answer the diagnostic question
- Depending on patient clinical scenario, low SAR protocols may be performed
- 1.5T static field strength
- Normal Operating Mode with SAR (≤1.5 or ≤ 2 W/kg) and dB/dt limitations (<200T/m/s)
- Coil limitations
- Body coil can be used for RF transmission
- Local transmit/receive coils if not positioned directly over the device
Workflow for non-MRI-conditional cardiac pacemakers/ICDs
- Prior to scheduling, device and leads identified and assessed for conditional/non-MRI-conditional status by any one of these roles: UCSF Implant vetting team, principal/senior MR Technologist, MR Technologist Supervisor or MRI MD.
- MD radiologist approval (QC fellow or section MRI safety representative) re: appropriateness to proceed – study indicated, presence of reasonable non-MRI alternative.
- Dependency/nondependency is evaluated per electrophysiology (EP) recommendations from pre-scan visit.
- Consent is obtained by radiology MD.
- EP Nurse Practitioner, EP Physician or EP Registered Nurse must interrogate and reprogram device at the time of scan.
- Monitoring for pacemaker dependent patients is performed by an EP nurse. Monitoring for pacemaker nondependent patient is performed by a trained radiology nurse.
- EP Nurse Practitioner, EP Registered Nurse or EP physician verifies pacemaker function following completion of the scan.
For non-MRI-conditional devices, risk-benefit analysis should clearly show the benefits of MRI. The study should not be performed if similar clinical information could be obtained with another image modality.
Assessment by EP (either inpatient consult or appointment with the EP clinic) should be schedule prior to the MRI date to review the details of the pacer/ICD and leads, and confirm that MRI is appropriate. EP should document in their notes regarding the encounter that a full discussion of risks and benefits of MRI was performed with the patient.
Device programming
Cardiac implanted electronic devices should be interrogated both before and after Cardiovascular Magnetic Resonance Imaging, with involvement of either electrophysiology providers or device representatives depending on the conditional/non-MRI-conditional status of the device. The device should be reprogrammed for the MRI including setting in either non-pacing or asynchronous pacing mode. Defibrillator therapies will be turned off, and magnet mode will be disabled. The device will be reprogrammed at the end of the exam.
Cardiac Loop Recorders
Cardiac Loop Recorders are MRI conditional devices. Cardiac Loop Recorders typically contain no lead wires or large loops of electrically conductive material. Product details regarding the specific loop recorder in question should be reviewed prior to MRI.
The main issue to consider with MRI is that data stored on the device may be altered or erased. Data should be downloaded before the time of study, and this issue should be discussed with the cardiologist who manages the loop recorder.
Because loop recorders contain ferromagnetic components, patients may feel slight movement during scanning. While not a safety hazard, patients should be made aware of this before scanning.